INTRODUCTION
Helicobacter pylori is recognized as a cause of chronic active gastritis, gastric and duodenal ulcers, and gastric cancer, though the mechanisms of pathogenesis for H. pylori-associated diseases are not yet well understood [1-4]. The ecological niche to which H.pylori is well-adapted is the mucous layer of the human gastric antrum, which has mucin glycoproteins as major constituents. Mucins, high-molecular weight carbohydrate-rich glycoproteins that coat the surface of the stomach and are secreted into the lumen, function to protect the stomach and could be important in H. pylori colonization. For further understanding the pathogenesis of H.pylori related diseases, it is important to consider whether H.pylori colonization of the surface epithelium is associated, as cause or effect, with changes in the gastric mucin synthesized by surface mucous cells.
MUCINS PRODUCED IN NORMAL STOMACH
The entire gastrointestinal tract is coated with a protective mucous layer. The main components of the viscoelastic mucous are mucin glycoproteins. Mucins are thought to protect the surface of the gastrointestinal tract from mechanical damage, from dessication, and from chemical irritants. Gastric mucins are the major components of an unstirred mucous-bicarbonate layer that protects the gastric epithelium from the high concentrations of acid in the stomach lumen and from autodigestion by pepsin. The protective functions of the gastric mucous layer imposes rigid requirements on the structure of gastric mucins (Figure 1). They must be very high in molecular weight and highly hydrated to provide the viscoelasticity necessary for protection from mechanical damage, and must also be acid-stable and have little non-glycosylated polypeptide exposed as a target for pepsin.
Figure 1.

Models of gastric mucin structure. Lower bars represent cDNA sequences of MUC1 cell-surface mucin and MUC5AC secreted mucin, with different domains labeled. Resulting structures of the proteins with attached carbohydrate are schematically represented above.
Like mucins from a number of sources, human gastric mucins are very high in molecular weight and are heavily substituted with O-linked oligosaccharides. Human mucins are encoded by at least nine distinct mucin genes, of which three, MUC1, MUC5AC, and MUC6, are expressed at high levels in the normal stomach (Table 1).
Table 1.
Human mucin genes
| Gene | Locus | mRNA size | Tandem Repeats | Other structural features | Expression in normal tissues |
| MUC1 | 1q21-24 | 4-7 kb | 20 AA | Transmembrane | Most epithelia |
| MUC2 | 11p15.5 | 14-16 kb | 23 AA, -16 AA | D-domains, cystine knot | Colon > small intestine > respiratory tract |
| MUC3 | 7q22 | 16-17.5 kb | 17 AA, 59 AA | Cystine knot | Small intestine > colon, gall bladder |
| MUC4 | 3q29 | 16.5-24 kb | 16 AA | Respiratory tract, cervix > GI tract | |
| MUC5AC | 11p15.5 | 17-18 kb | 8 AA | D-domains, cystine knot | Stomach (surface) > respiratory tract |
| MUC5B | 11p15.5 | 17.5 kb | 29 AA, interrupted | D-domains, cystine knot | Respiratory tract, salivary gland, cervix > GI tract |
| MUC6 | 11p15.5 | 16.5-18 kb | 169 AA | Cystine knot | Stomach (glands), gall bladder |
| MUC7 | 4 | 2.4 kb | 23 AA | No homology to large mucins | Salivary glands |
| MUC8 | 12q24.3 | -9 kb | 18 AA, 41 AA | Not Thr/Ser rich | Trachea |
MUC1 mucin is well characterized[5]. The protein encoded by the MUC1 gene has a large central domain (VNTR, Variable Number of Tandem Repeats) composed of a variable number (25 to 125) of tandem repeats of a 20-amino acid sequence with 25% threonine and serine and 7% proline. Unlike most other mucins, the MUC1-encoded protein has a transmembrane segment and a cytoplasmic tail that can interact with the cytoskeleton. The O-linked carbohydrates on MUC1 mucin are heterogeneous, differ between tumors and normal epithelial cells[6], and can influence the recognition of the mucin protein by different monoclonal antibodies. MUC1 mucin is produced to some extent by most epithelial cells, but has been studied most extensively in mammary, pancreatic, and colon cancer cells.
MUC5AC is expressed in the stomach and in tracheobronchial cells. Immunohistochemical studies indicate that MUC5AC apomucin is present in surface mucous cells of the gastric epithelium[7,8]. MUC5AC mucin has a small tandem repeat sequence of 8 amino acids, interspersed with cysteine-containing regions(Figure 1). The glycoprotein is very large with the bulk of the molecule made up of heavily glycosylated tandem repeats of an 8-amino acid peptide sequence rich in threonine (to which O-linked oligosaccharides are attached) and proline. Interspersed irregularly within the tandem repeat region are cysteine-containing motifs. Like MUC2 intestinal mucin, both the N-terminal and the C-terminal have cysteine-rich globular domains[9] with sequence similarity to the D domains of pre-pro-von Willebrand factor and an inferred cystine-knot motif, topologically similar to epidermal growth factor (EGF). The cysteine-rich globular domains of secreted mucins may be involved in the oligomerization of mucin or in binding to collagen of basement membranes[10]. MUC5AC is expressed in the normal stomach, but not in normal colon. Aberrant expression of MUC5AC has been reported, however, in colorectal cancers and adenomas[8,11,12] and may be related to the progression of colon cancers.
MUC6 is also expressed in the normal stomach, but in mucous glands rather than surface mucous cells[7,8,13,14]. This mucin has a very large tandem repeat sequence of 169 amino acids, very high in amounts of Thr, Ser, and Pro[13]. The high content of Thr and Ser likely accounts for the large amount of carbohydrate present on this molecule. Thus, there are (at least) two completely different secreted mucins produced by the normal stomach. How these mucins differ in carbohydrate (and whether they differ in function) is speculative. Genes for both MUC5AC and MUC6 have been assigned to a region of chromosome 11 that also codes for two other secretory mucins[15], with a gene order of HRAS-MUC6-MUC2-MUC5AC-MUC5B-IGF2.
Like mucins from other sources, the carbohydrate portion of gastric mucin is heterogeneous. Several neutral oligosaccharide structures have been published [16,17], but the overall complement of oligosaccharides is not known. The results of histochemical studies suggest that the carbohydrate portion of the mucin in surface mucous cells is different from that in mucous glands. The surface mucous cells are stained by PAS, while mucous glands (cardiac gland, mucous neck cells, and pyloric gland cells) are stained by alcian blue. The “neutral” mucins in surface mucous cells are also stained by galactose oxidase/Schiff, suggesting the presence of terminal Gal or GalNAc[18]. The mucous gland cells uniquely show periodate-enhanced binding of concanavalin A, called paradoxical ConA staining. The structural basis for this is unclear, but it may detect terminal alpha-GlcNAc[18]. The mucous gel layer covering the surface epithelium has been shown to have clearly demarcated layers of two distinct mucin types[19]. On the basis of staining properties, these likely correspond to the surface-type neutral mucin (MUC5AC) reactive with galactose oxidase and to the gland-type acid mucin (MUC6), possibly sulfomucin, revealed by periodate-enhanced (paradoxical) ConA staining.
EFFECT OF H.pylori ON GASTRIC MUCIN IN VIVO
Though both the thickness and the hydrophobicity of the mucous gel layer is decreased in the gastric mucosa of ulcer patients[20], it has not been established whether this is associated with increased mucin degradation, decreased mucin synthesis, or a change in mucin type. It has been postulated that one important pathogenic property of H.pylori is its ability to weaken the mucous gel that protects the gastric epithelium[21,22], but the presence or absence of mucinase activities in H.pylori is controversial[23-25]. Direct analysis of mucins from H.pylori infected and uninfected patients show no decrease in viscosity, arguing against H.pylori dependent mucin degradation [26] . In spite of histochemical observations of mucous dep letion accompanying H.pylori infection, qualitative alterations in the type of mucin produced have only recently been studied.
In order to determine the effect of H.pylori infection on mucin gene expression in the gastric epithelium[7], biopsies from H.pylori-positive and H. pylori negative patients were examined by immunohistochemistry (Table 2). MUC6 was limited to mucous glands of H.pylori-negative patients, but 72% of H.pylori positive patients also expressed MUC6 on surface muco us cells. In contrast, MUC5AC mucin was seen in significantly fewer surface muco us cells of H.pylori-positive specimens. Overall, the percent of the surface epithelium stained by anti-MUC5 was significantly lower in H.pylori-positive specimens than in H.pylori-negative specimens (P < 0.01). Carbohy drates recognized by LeX and paradoxical ConA staining were aberrantly expressed in the surface mucous cells of 16/27 and 17/23 of H.pylori-positive tissu es, respectively. There was a suggestive but non-significant decrease in staini ng for MUC1 mucin. Retrospective examination of clinical histories and histologi cal findings showed that the mucin alterations occur in H.pylori infected individuals with and without ulcers, but not in patients with non-H.pylori-associated gastritis or gastric ulcers (Figure 2). This indicates that the muc in alterations are not simply a secondary effect of inflammation.
Table 2.
Histochemical staining of mucins in gastric biopsy specimens
| Antigen | H.pylori negative | H.pylori positive | |
| MUC5AC | % Stained*a, intensity score (surface) | (69.8% ± 3.5%), 2.8 ± 0.1 | (51.2% ± 5.7%), 2.6 ± 0.1 |
| MUC6 | % Stained**a, intensity score (surface)a | 4%, 0.1 ± 0.1 | 72%, 1.8 ± 0.2 |
| Le\ + b | % Stained**a, intensity score (surface)a | 63%, 0.9 ± 0.2 | 96%, 1.9 ± 0.2 |
| Paradoxical ConA | % Stained**a, intensity score (surface)a | 18%, 0.4 ± 0.2 | 79%, 2.1 ± 0.3 |
| LeX | % Stained**a, intensity score (surface)a | 4%, 0.4 ± 0.4 | 59%, 1.0 ± 0.2 |
| MUC1 | Intensity score (surface), intensity score(glands) | 2.6 ± 0.2, 1.7 ± 0.1 | 2.7 ± 0.1, 1.4 ± 0.2 |
| Sialyl Tn | Intensity score (surface), intensity score (glands)a | 1.8 ± 0.2, 1.9 ± 0.1 | 1.6 ± 0.1, 1.4 ± 0.1 |
% of surface epithelium stained; **% of patients with surface staining;
P < 0.05, H.pylori positive vs H.pylori negative
Figure 2.
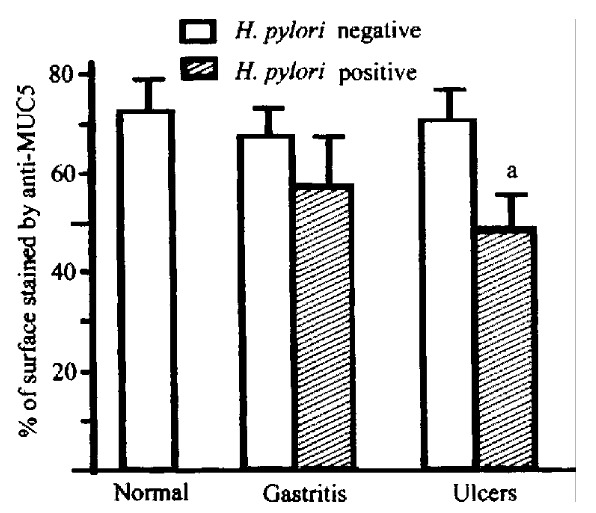
Expression of MUC5AC in surface epithelium of normal human stomach and gastritis and gastric ulcer specimens. aP < 0. 05 vs corresponding H. pylori negative group.
For more direct examination of mucin gene expression, the presence of MUC5 AC and MUC6 message in antral biopsies were examined by in situ hybridization[7]. In antral biopsy specimens from H. pylori-negative patients, MUC5AC mRNA was homogeneously expressed in surface epithelial cells. MUC5AC exp ression in the surface epithelial cells of H.pylori -positive patients was patchy, however, and often absent from large areas of the surface epithelium. Co ncordance between the pattern of MUC5AC expression as determined by in situ hybridization and immmuno-histochemistry was 100%. MUC6 expression was limited to cells of the deep glands in H.pylori -negative patients, seen by both in situ hybridization and immunohisto-chemistry. In contrast, 6 of 8 H.pylori-positive specimens (0/7 H.pylori negative specimens) had focal MUC6 mRN A expression in surface epithelial cells. MUC5AC and MUC6 gene expression were e xamined in antral biopsies obtained from patients with H.pylori -associated antral gastritis (biopsy-proven) before and after documented eradication of th e bacterium. In 7 of 10 cases MUC5AC expression increased (P = 0.004) after H.pylori eradication (Figure 3). Eradication of H.pylori also resulted in reversal of MUC6 antigen expression toward normal patterns.
Figure 3.
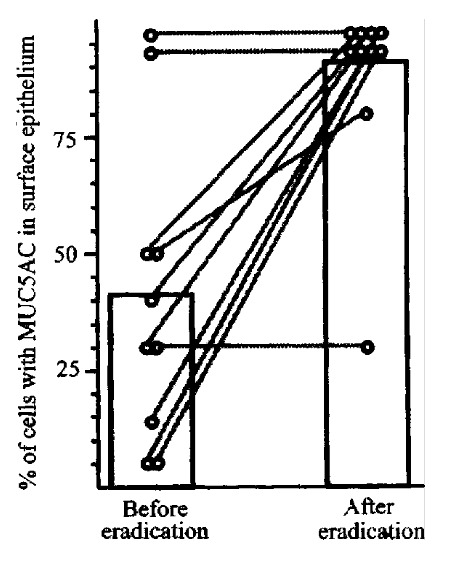
MUC5 expression in patients before and after eradication of H.pylori infection. MUC5 gene expression was determined by in situ hybridization.Bars show mean percent of epithelial cells expressing MUC5. Lines show changes in MUC5AC expression in individual patients.
The effect of H.pylori on gastric mucin expression was further examined by purification and immunochemical analysis of mucins from gastric juice of H. pylori-positive and H.pylori-negative patients. For H.pylori-infe cted patients and uninfected patients that had been examined for immunohistochemical staining of biopsy tissues, gastric aspirates were used as a source for muc in purification by gel filtration and CsCl density gradient centrifugation. Ther e was no significant difference in yield of mucin or carbohydrate content betwee n H.pylori -positive and H.pylori negative specimens (Figure 4). The p urified mucins were examined by ELISA for MUC6 and Le-b antigenic activity. MUC 6 activity was higher (P = 0.026) in mucins from the H.pylori-infected p atients than in mucins from uninfected patients. Mucins from the H.pylori - infected patients also bound monoclonal antibody to Le-b antigen to a significa ntly greater extent (P = 0.014) than mucins from the uninfected patients (Figure 4). Subsequently, these purified mucins have been examined by SDS-PAGE and Western analysis. Infection with H.pylori was associated with an increase in MUC6 (detected with anti-M6P and also with anti-Le-b and Ulex europea aggl utinin) and a decrease in MUC5AC (detected with anti-M5P and 45M1 antibodies and also with peanut agglutinin and Vicia villosa agglutinin) in these secre ted mucins.
Figure 4.
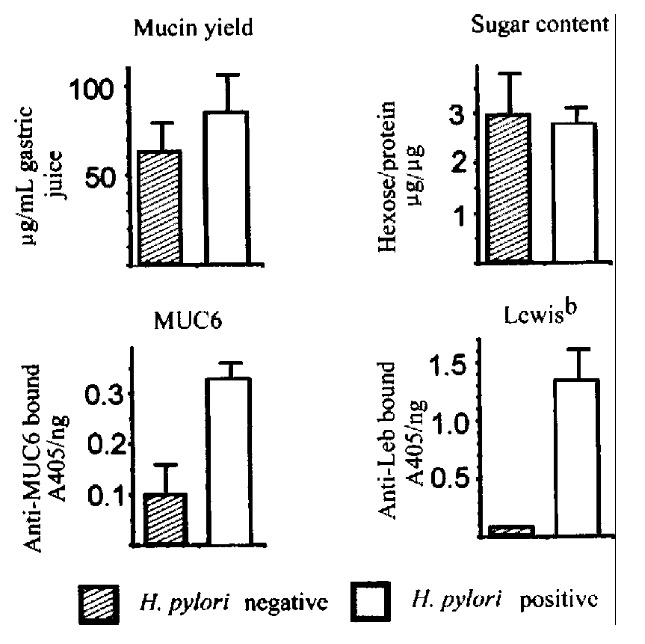
Purification and analysis of mucins from gastric juice. Upper left, yield of mucins purified from gastric aspirates of 5 H. pylori -negative and 5 H.pylori -positive patients. Upper right, carbohydrate content of purifi ed mucins. Lower left, binding of antibody to MUC6 peptide in ELISA. Lower right , binding of antibody to Lewis-b antigen.
These results establish that there is aberrant surface expression of gland-type gastric mucin in surface mucous cells of H.pylori infected patients, acc ompanied by focally decreased MUC5AC mucin. This decrease in MUC5AC mucin and ab errant expression of MUC6 might be expected to disrupt the protective surface mu cinlayer. How or whether alterations in gastric mucins would influence processes that lead to disease is an important question which requires that the specific ity and mechanisms of mucin depletion be better understood.
EFFECT OF H.pylori ON MUCIN SYNTHESIS IN VITRO
Analyses of tissue specimens and purified mucin glycoproteins indicate that gastric surface-type mucin expression is reversibly decreased in H.pylori-infected patients[7] but do not allow direct examination of mucin synthesis. Gastric cells in culture were examined to determine the effect of H .pylori on mucin synthesis[27]. KATO III gastric epithelial cells were incubated in the presence or absence of H. pylori, and the mucin produced was quantitated by labeling with[3H]glucosamine and size exclusion HPLC on Superose 6 columns. The 3Hlabeled high-molecular weight glycop rotein was confirmed to be mucin by CsCl density gradient centrifugation, chemical and enzymatic degradation treatments. H . pylori (type strain NCTC11637), under conditions that had little effect on viability, inhibited the synthesis of mucin by 82% (Figure 5). There was no inhibition of mucin synthesis by the non -gastric pathogen Campylobacter jejuni, and little inhibition by a strain ( Tx30a) of H.pylori that is CagA-negative and non-toxigenic. Similar results were seen in five other gastric cell lines tested (Figure 5). Inhibition of m ucin synthesis was detected as early as 4h after addition of bacteria, and was partially reversible, though with a slower time-course than the onset of inhibi tion. Inhibition of mucin labeling was concentration dependent (Figure 6) and did not require the presence of intact bacteria. There was no inhibition by a solu ble extract of H.pylori, but the H.pylori pellet fraction gave inhibit ion equivalent to intact bacteria.
Figure 5.
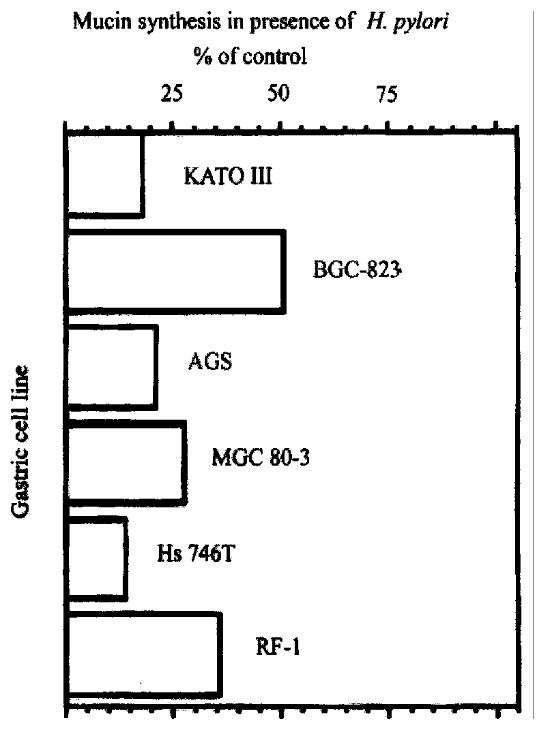
Effect of H.pylori on mucin synthesis in six gastric cell lines. Cells were labeled 22 h with [3H] glucosamine in the presence or absence of 1 OD600 H.pylori, and labeled glycoproteins were analyzed by size-exclusion HPLC.
Figure 6.
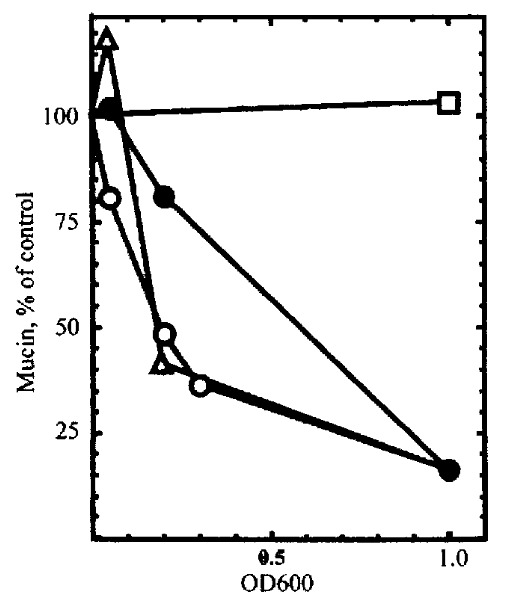
Inhibition of mucin synthesis by subfractions of H.pylori. KATO I II cells were labeled with [3 H]glucosamine in the presence of different concentrations of intact H.pylori (open circles), H.pylori l ysate (filled circles), the 100000 × g pellet (open triangles), or the 100000 × g supernatant (open squares).
In a pulse-chase analysis, H.pylori had no effect on mucin secretion. Fur thermore, there was little or no degradation of mature mucin in the presence or absence of H.pylori. Further experiments, to examine the effects of H.pylori on mucin glycosy lation, used benzyl-GalNAc, which specifically inhibits synthesis of peripheral carbohydrate on mucin-type glycoproteins[28]. Incubation of KATO III with H.pylori decreased labeling of mucin to a simil ar extent in the presence or absence of benzyl-GalNAc (Figure 7), indicating th at the effect of H.pylori is not due to inhibition of peripheral glycosylat ion per se, but results from inhibition of synthesis of mucin core structures.
Figure 7.
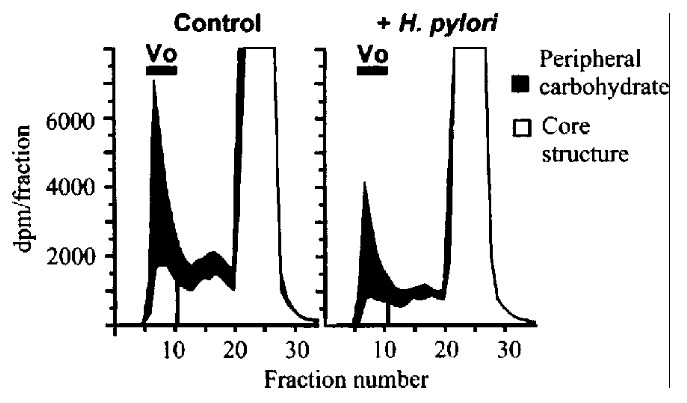
Effect of H.pylori on synthesis of peipheral and core carbohydrat e structures of mucin. KATO III cells were incubated with or without 2 mmol/L b enzy l-GalNAc, and labeled for 4 h in the presence or absence of 1 OD600 H.pylori . Bars show void volume (Vo) containing labeled mucin. Solid areas show labeli ng of the peripheral carbohdyrate (inhibitable by benzyl-GalNAc). Open areas sh ow residual labeling of core structures in the presence of benzyl-GalNAc.
KATO III produces MUC5AC and MUC1 mucins, and the amount of both mucin pro teins is decreased by co-incubation with H. pylori (Figure 8) . Expression of another high molecular weight glycoprotein, CEA, and another control protein, galectin-3, was unaffected by H.pylori. H.pylori also decreased the a mount of MUC5AC protein in BGC-823 gastric cells and the amount of MUC1 protein in the BGC-823, AGS, and MGC 80-3 cell lines. The inhibition of synthesis of both MUC5AC and MUC1 protein was concentr ation dependent and associated with the insoluble fraction of H. pylori lysates. Kinetically, the onset of inhibiti on of MUC1 expression was more rapid than inhibition of MUC5AC expression. MUC1 inhibition was seen within 4 h while MUC5AC inhibition was slower. MUC1 recovery was also more rapid than recovery of MUC5AC.
Figure 8.
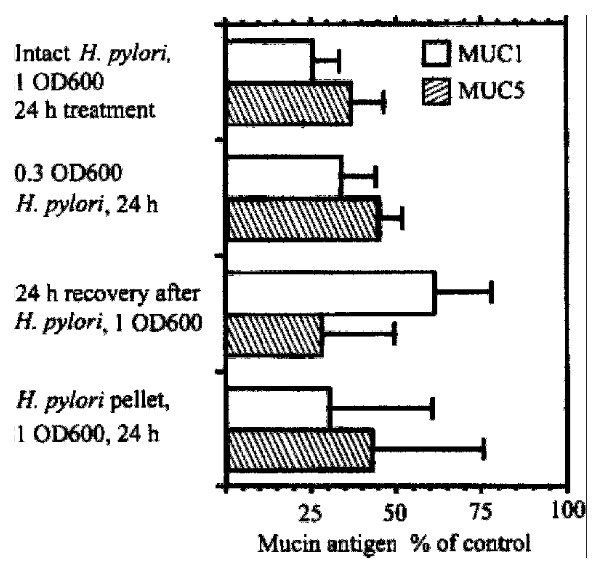
Effect of H.pylori on expression of MUC1 and MUC5AC in KATO III gastric epithelial cells in vitro. KATO III cells were incubated with or with out H.pylori, and cell lysates were subjected to Western analysis. Content of MUC1 antigen, detected with monoclonal antibody HMFG2, and of MUC5AC antigen, detected with monoclonal antibody CLH2, is expressed as percentage of untreated cells.
These experiments[27] demonstrate that H.pylori decreases the amou nt of total mucin, and MUC5AC and MUC1 proteins in gastric epithelial cells. Ind irect evidence indicates that this is due to a decrease in the synthesis of mucin protein rather than changes in glycosylation, secretion, or degradation of muc ins. These results may help to explain the mucin depletion associated with H. pylori infection in vivo[7,29].
INFLUENCE OF MUCINS ON H.pylori ADHESION
Most of the H.pylori in the stomach are present in the mucus gel layer, and appear to cause little harm to the host; adhesion of H.pylori to the gas tric epithelial cell surface may be required for causing disease. For example, induction of the proinflammatory chemokine interleukin-8, requires that bacteria be in contact with the epithelial cell surface[30,31]. Mucin glycoprot eins produced by the gastric epithelial surface could influence the process of H.pylori adhesion in two ways: First, secreted glycoproteins could bind to bacterial adhesins and help to keep the bacteria in the mucous gel layer, preventing their approach to the epithelium. Although H.pylori has several differ ent adhesins which could be involved in binding to mucins[32-35] and human gastric mucin has been shown to inhibit bacterial binding to other cell types, e .g., erythrocytes and HEp-2 cells[36,37], it is not known whether secr eted gastric mucin can inhibit the adhesion of H. pylori to gastric epithelial cells . Second, cell-surface mucin glycoproteins could shield the epithelial cell surface from exposure to contact-dependent virulence factors, preventing adhesion-dependent synthesis of pro- inflammatory chemokines. In MUC1-expressing cells, the highly glycosylated tandem repeat domain extending out from the cell surface can interfere with cell-cell interactions, for example, integrin-med iated aggregation[38]. Since adhesion of H.pylori to gastric epithe lial cells requires their close proximity to the cell surface where they can int eract with integrins or other cell-surface receptors, cell-surface mucins migh t be expected to block adhesion of H.pylori to gastric epithelial cells.
Since previous results indicated that gastric surface-type mucins are decr eased by H.pylori both in vivo[7]and in vitro[27] , we sought to determine the influence of mucin on adhesion of H.pylori to cultured gastric epithelial cells. For measurement of the adhesion of H.pylori to gastric epithelial cells, an assay was established using biotinylated H .pylori, with bacteria attached to the BGC-823 gastric epithelial cells quan titated with avidin-biotin-peroxidase complex and ABTS as chromogen. The binding of bacteria was characterized with regard to time dependence, temperature dep endence, and bacterial strain dependence. Optimal conditions for adhesion were found to be 30 min incubation at 37 °C. Under conditions where the CagA/cytotoxin positive type strain of H.pylori, NCTC 16137, bound well to BGC-823 cells, there was little binding of the CagA-negative, cytotoxin-negative strain of H. pylori, Tx30a or of the non-human pathogen Helicobacter mustelae. As further validation, the standard binding assay was compared to colony counts for detection of viable H.pylori bound to BGC-823 cells (Figure 9). Binding of biotinylated bacteria was equivalent to binding of viable bacteria.
Figure 9.
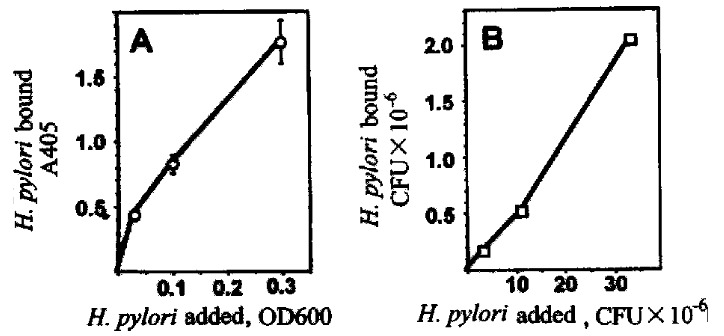
Assay of adhesion of H.pylori to gastric epithelial cells. Biotin -labeled H.pylori were incubated with BGC-823 cells for 30 min at 37 °C, and attached bacteria were quantitated using the avidin-biotin-complex assay (A, left panel) or by colony counts (B, right panel).
BGC-823 cells are well differentiated, attach well to tissue culture plastic, and are susceptible to H.pylori-dependent inhibition of mucin synthesis[27,39]. In comparison to other gastric epithelial cell lines, there w as more binding of H.pylori to BGC-823 cells (which produces MUC5AC mucin and has relatively low levels of MUC1 cell - surface mucin) than to two cell lines (AGS and MGC-803) which do not produce MUC5AC mucin but produce high levels o f MUC1 mucin[27,40]. This inverse correlation between MUC1 expression and H.pylori adhesion in gastric epithelial cell lines suggests that MUC1 mucin could interfere with H.pylori adhesion.
In order to test the role of mucin in H.pylori adhesion, we sought to inh ibit mucin synthesis in BGC-823 gastric epithelial cells and examine the effect on binding of bacteria. It was initially confirmed that treatment of BGC-823 cells with the mucin-specific glycosylation inhibitor benzyl-GalNAc inhibits to tal mucin synthesis (measured by labeling with [3H] glucosamine and size-exclusion chromatography) by approximately 80% (Figure 10). Treatment of 823 cells with benzyl-GalNAc significantly increased the adhesion of H. p ylori (treated/control = 1.43 ± 0.14, n = 6). These results indicate that inhib ition of mucin glycosylation is associated with an increase (rather than a decre ase) in H.pylori adhesion, suggesting that mucins protect against (rather t han facilitate) binding of the bacterium to the gastric epithelial surface.
Figure 10.
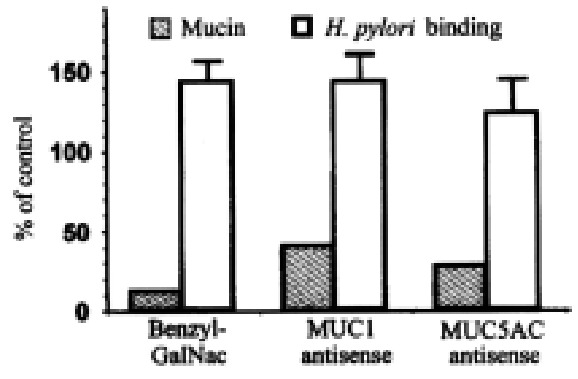
Effect of inhibitors of mucin synthesis on H.pylori adhesion. BG C-823 cells were treated with 2 mmol/L benzyl-GalNAc, with 5 μmol/L MUC1 antisense (5’-GGG-CTG-GGG-GGG-CGG-TGG-3’), or with 5 μmol/L MUC5AC antisense (5’-AGA-GGT-TGT-GCT-GGT-TGT-3’). Solid bars show, as percent of control, amo unts of total mucin (left), MUC1 mucin (middle), and MUC5AC mucin (right). Open bars show binding of biotinylated H.pylori, expressed as percent of control .
In order to specifically decrease the synthesis of MUC1 and MUC5AC phospor othiolate antisense oligodeoxynucleotides were designed and targeted against th e VNTR regions (M1TR) of MUC1 and MUC5AC genes. By Western analysis (Figure 10) , MUC1 antisense treatment decreased MUC1 protein (treated/control = 0.39 ± 0.15, n = 6), and MUC5AC antisense treatment decreased MUC5AC protein (treated/control = 0.27 ± 0.13, n = 3). MUC1 antisense oligodeoxynucleotide treatment of BG C-8 23 cells significantly increased the adhesion of biotinylated H.pylori, (treated/control = 1.43 ± 0.18, n = 4). MUC5AC oligodeoxynucleotide treatment ha d no significant effect on adhesion (treated/control = 1.23 ± 0.22, n = 5), but it sh ould be noted that the culture medium, which would contain most of the secreted (MUC5AC) mucins, was removed before the adhesion assay was performed.
These results indicate that cell-surface mucin glycoprotein decreases adhe sion of H.pylori to gastric epithelial cells. Since inhibition of mucin syn thesis in vitro is associated with an increase in H. pylori adhesion, H . pylori - dependent mucin depletion in vivo would be expected to facilitate further binding of the bacterium to the gastric epithelial surface.
CONCLUSION AND WORKING HYPOTHESIS
Our working hypothesis (Figure 11) is that H. pylori alters the synthesis of gastric mucin in surface mucous cells and that the resultant alteration in the surface mucous gel layer facilitates adhesion of H.pylori to the epithelial cell surface, which could lead to increased inflammation. Based on in vitro results, H.pylori adhesion decreases mucin synthesis, and decreased mucin synthesis increases H.pylori adhesion. If asimilar cycle applies in vivo, the pathogenetic effects of H.pylori infection could be mechanistically tied to the mucin depletion observed histologically.
Figure 11.
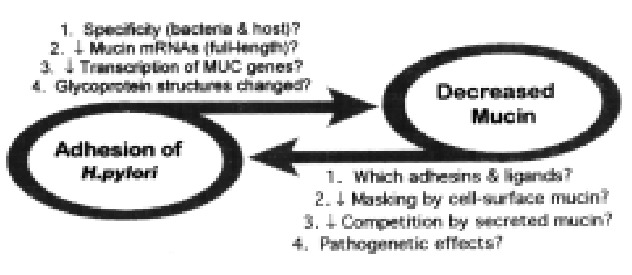
Working hypothesis: interaction of H.pylori and gastric mucins.
The diseases caused by H.pylori, gastroduodenal ulcers and gastric cancer, take years to develop, but only short-term effects can be studied in vitro. H. pylori infection causes little overt damage to the gastric epithelium in most of the host population. H.pylori may, therefore, cause ulcers thro ugh a subtle disturbance in the interaction between the bacteria and the host, r ather than acute damage to one critical component of the host cell. Non-physiol ogical conditions are necessary for observable effects in cultured cell systems. Caution must therefore be exercised in extrapolating these data to the in vivo interactions between H.pylori adhesion and mucin expression. Neverthele ss, if, as we hypothesize, H.pylori adhesion to gastric epithelial cells de creases mucin synthesis, and decreased mucin synthesis further increases H.pylori adhesion, even small effects on both processes could eventually lead to d isease.
Footnotes
Supported by the Research Service of the Henry Ford Health Sciences Center and Research Foundation (JCB, RSB) and National Cancer Institute Grant R01 CA69480 (R-SB)
Edited by Lu J proofread by Mittra S
References
- 1.Veldhuyzen van Zanten SJ, Sherman PM. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic overview. CMAJ. 1994;150:177–185. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee A. The microbiology and epidemiology of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:2–6. [PubMed] [Google Scholar]
- 3.Blaser MJ, Parsonnet J. Parasitism by the "slow" bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 6.Brockhausen I, Yang JM, Burchell J, Whitehouse C, Taylor-Papadimitriou J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607–617. doi: 10.1111/j.1432-1033.1995.607_2.x. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- 8.Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 9.Gum JR, Hicks JW, Toribara NW, Rothe EM, Lagace RE, Kim YS. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992;267:21375–21383. [PubMed] [Google Scholar]
- 10.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 11.Buisine MP, Janin A, Maunoury V, Audié JP, Delescaut MP, Copin MC, Colombel JF, Degand P, Aubert JP, Porchet N. Aberrant expression of a human mucin gene (MUC5AC) in rectosigmoid villous adenoma. Gastroenterology. 1996;110:84–91. doi: 10.1053/gast.1996.v110.pm8536891. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Sternberg L, Yan P, Ho SB, Bresalier RS. Ectopic expres-sion of MUC5 gastric mucin in colorectal adenocarcinoma. Gastroenterology. 1998;114:A573. [Google Scholar]
- 13.Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, Gum JR, Byrd JC, Siddiki B, Kim YS. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- 14.De Bolós C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 15.Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d'Hooge MC, Laine A, Van-Seuningen I, Degand P, Gum JR, et al. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38:340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- 16.Slomiany A, Zdebska E, Slomiany BL. Structures of the neutral oligosaccharides isolated from A-active human gastric mucin. J Biol Chem. 1984;259:14743–14749. [PubMed] [Google Scholar]
- 17.Slomiany BL, Zdebska E, Slomiany A. Structural characterization of neutral oligosaccharides of human H+Leb+ gastric mucin. J Biol Chem. 1984;259:2863–2869. [PubMed] [Google Scholar]
- 18.Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y. A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J. 1991;23:22–28. doi: 10.1007/BF01886504. [DOI] [PubMed] [Google Scholar]
- 19.Ota H, Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J. 1992;24:86–92. doi: 10.1007/BF01082444. [DOI] [PubMed] [Google Scholar]
- 20.Spychal RT, Goggin PM, Marrero JM, Saverymuttu SH, Yu CW, Corbishley CM, Maxwell JD, Northfield TC. Surface hydrophobicity of gastric mucosa in peptic ulcer disease. Relationship to gastritis and Campylobacter pylori infection. Gastroenterology. 1990;98:1250–1254. doi: 10.1016/0016-5085(90)90341-w. [DOI] [PubMed] [Google Scholar]
- 21.Sarosiek J, Peura DA, Guerrant RL, Marshall BJ, Laszewicz W, Gabryelewicz A, McCallum RW. Mucolytic effects of Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;187:47–55. [PubMed] [Google Scholar]
- 22.Slomiany BL, Slomiany A. Mechanism of Helicobacter pylori pathogenesis: focus on mucus. J Clin Gastroenterol. 1992;14 Suppl 1:S114–S121. [PubMed] [Google Scholar]
- 23.Suerbaum S, Friedrich S. Helicobacter pylori does not have a hap mucinase gene that is quasi-identical to the Vibrio cholerae hap gene. Mol Microbiol. 1996;20:1113–1114. doi: 10.1111/j.1365-2958.1996.tb02551.x. [DOI] [PubMed] [Google Scholar]
- 24.Baxter A, Campbell CJ, Cox DM, Grinham CJ, Pendlebury JE. Proteolytic activities of human Campylobacter pylori and ferret gastric Campylobacter-like organism. Biochem Biophys Res Commun. 1989;163:1–7. doi: 10.1016/0006-291x(89)92089-5. [DOI] [PubMed] [Google Scholar]
- 25.Sidebotham RL, Batten JJ, Karim QN, Spencer J, Baron JH. Breakdown of gastric mucus in presence of Helicobacter pylori. J Clin Pathol. 1991;44:52–57. doi: 10.1136/jcp.44.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markesich DC, Anand BS, Lew GM, Graham DY. Helicobacter pylori infection does not reduce the viscosity of human gastric mucus gel. Gut. 1995;36:327–329. doi: 10.1136/gut.36.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd JC, Yunker CK, Xu QS, Sternberg LR, Bresalier RS. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology. 2000;118:1072–1079. doi: 10.1016/s0016-5085(00)70360-x. [DOI] [PubMed] [Google Scholar]
- 28.Byrd JC, Dahiya R, Huang J, Kim YS. Inhibition of mucin synthesis by benzyl-alpha-GalNAc in KATO III gastric cancer and Caco-2 colon cancer cells. Eur J Cancer. 1995;31A:1498–1505. doi: 10.1016/0959-8049(95)00248-h. [DOI] [PubMed] [Google Scholar]
- 29.Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, Graham DY, Genta RM. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433:419–426. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 30.Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieder G, Hatz RA, Moran AP, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doig P, Austin JW, Kostrzynska M, Trust TJ. Production of a conserved adhesin by the human gastroduodenal pathogen Helicobacter pylori. J Bacteriol. 1992;174:2539–2547. doi: 10.1128/jb.174.8.2539-2547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans DG, Evans DJ, Moulds JJ, Graham DY. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alkout AM, Blackwell CC, Weir DM, Poxton IR, Elton RA, Luman W, Palmer K. Isolation of a cell surface component of Helicobacter pylori that binds H type 2, Lewis(a), and Lewis(b) antigens. Gastroenterology. 1997;112:1179–1187. doi: 10.1016/s0016-5085(97)70129-x. [DOI] [PubMed] [Google Scholar]
- 35.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 36.Tzouvelekis LS, Mentis AF, Makris AM, Spiliadis C, Blackwell C, Weir DM. In vitro binding of Helicobacter pylori to human gastric mucin. Infect Immun. 1991;59:4252–4254. doi: 10.1128/iai.59.11.4252-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piotrowski J, Slomiany A, Murty VL, Fekete Z, Slomiany BL. Inhibition of Helicobacter pylori colonization by sulfated gastric mucin. Biochem Int. 1991;24:749–756. [PubMed] [Google Scholar]
- 38.Van Klinken BJ, Dekker J, Büller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 39.Cai YH, Zhi GY, Zhang JQ, Ping L, Zhou QH. The establishment of human ga stric cancer cell line BGC-823. J Beijing Medical Univ. 1986;18:136–139. [Google Scholar]
- 40.Dahiya R, Kwak KS, Byrd JC, Ho S, Yoon WH, Kim YS. Mucin synthesis and secretion in various human epithelial cancer cell lines that express the MUC-1 mucin gene. Cancer Res. 1993;53:1437–1443. [PubMed] [Google Scholar]


