Abstract
Background/Aims
The primary aim of this study was to characterise the blood metabolic profile of hepatocellular carcinoma (HCC) in a rat model, and the secondary aim was to evaluate the effect of the quinolone, norfloxacin on metabolic profiles and exploring the role that gut sterilisation may have on HCC development.
Methods
HCC was induced in 10 Fischer rats by administration of intra-peritoneal diethylnitrosamine (DEN) and oral N-nitrosomorpholine. Plasma was collected upon sacrifice. Five of these rats were concomitantly administered oral norfloxacin. Six Fischer non-treated rats acted as healthy controls. Proton nuclear magnetic resonance (NMR) spectra were acquired using a 600 MHz NMR system.
Results
Control animals were 120 g heavier than diseased counterparts. Proton NMR spectra from diseased rats displayed significant decreases in lipoproteins, unsaturated fatty acids, acetyl-glycoprotein, acetoacetate, and glucose (P ≤ 0.001). Plasma citrate and formate levels were increased (P = 0.02). Norfloxacin appeared to abrogate this effect slightly.
Conclusion
The spectral profiles of plasma in rats with HCC display marked changes with relation to lipid metabolism and cellular turnover. Norfloxacin appears to moderate these metabolic alterations to a small degree.
Abbreviations: 1-D, one-dimensional; CPMG, Carr-Purcell-Meiboom-Gill 3B; DEN, diethylnitrosamine; FID, free induction decay; 1H, human proton; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NOESY, nuclear overhauser effect spectroscopy; NMOR, N-nitrosomorpholine; NMR, nuclear magnetic resonance; PCA, principal components analysis; PLS-DA, partial least squares discriminant analysis; Q2, goodness of prediction; R2, goodness of fit; RD, relaxation delay; RF, radiofrequency; SBP, spontaneous bacterial peritonitis; TLR-4, Toll-like receptor 4; VLDL, very low-density lipoprotein
Keywords: hepatocellular carcinoma, norfloxacin, metabonomics, NMR spectroscopy
Introduction
Human proton (1H) nuclear magnetic resonance (NMR) spectroscopy studies of blood in patients with hepatocellular carcinoma (HCC) have identified a number of altered metabolites, implicating changes in hepatic function, lipid metabolism and bile acid metabolism.1, 2 Many rat models of HCC exist, but there are no reports of 1H NMR spectroscopy studies of rat serum or plasma. Characterisation of the serum or plasma metabolic changes in an animal model of HCC would provide valuable translatable information to human disease.
There have been two reports of rat HCC tissue 1H NMR metabolite profiling studies. Tesiram and colleagues performed a meticulous study using 1H NMR spectroscopy to investigate the changes of lipid metabolites in a Fischer rat model of hepatic adenoma, induced with a choline deficient diet.3 It was demonstrated that a substantial increase in glycerol backbone-containing phospholipids increased throughout adenoma development. HCC did not develop in these animals, but changes described in adenomas are likely to share similarities with HCC. A second 1H NMR spectroscopy study, performed in male Sprague-Dawley rats with diethylnitrosamine (DEN)-induced HCC, reported raised low, very low and high-density lipoprotein (LDL, VLDL and HDL) levels in addition to alterations in branch chain amino acids, acetate, glutamine, choline, trimethylamine-N-oxide, glycine, glucose and glycogen.4 Whether these metabolic profiles are reflected in blood has not previously been investigated using 1H NMR spectroscopy.
Quinolones are administered to patients with decompensated cirrhosis for the treatment of spontaneous bacterial peritonitis (SBP). It has also been reported that quinolones improve survival and decrease incidence of other end-stage complications of liver disease, such as hepatorenal syndrome.5 Their effect might be mediated through a decrease in gut bacterial translocation and circulating endotoxin levels, reducing the pro-inflammatory effectors of liver fibrosis.6 Increased bacterial translocation, endotoxin and activation of the TLR-4 pathway have also been shown to promote inflammation and HCC.7 It has been suggested that antibiotic therapy may play a role in reducing HCC incidence in cirrhosis. Several studies of cirrhosis patients and animal models have shown gut sterilisation with antibiotics with quinolones, such as norfloxacin, can decrease bacterial translocation, circulating levels of endotoxin and hepatic inflammation.8, 9, 10, 11, 12 Therefore, the effect this class of drugs may have on HCC development is of interest.7 This has not been investigated previously.
The primary aim of this study was to ascertain whether altered metabolic profiles could be characterised in a rat model of HCC using 1H NMR spectroscopy. The secondary aim was to investigate the effect of the quinolone, norfloxacin on HCC-induced 1H NMR plasma profile changes.
Methods
Prior ethical approval was obtained from the University College Hospitals Local Research Ethics Committee, with animal treatment in accordance with the UK Animals (Scientific Procedures) Act 1986.
Fischer Rat Model of HCC
HCC was induced by the administration of two potent carcinogens, DEN (Sigma-Aldrich, Gillingham, UK) and N-nitrosomorpholine (NMOR) (Sigma-Aldrich, Gillingham, UK), both of which have rapid rates of hepatic metabolism. This model was chosen owing to the quick development time of HCC (within 16 weeks). However, a washout period of 2 weeks prior to sacrifice was instituted in order to minimise any residual metabolic effects of DEN and NMOR themselves. Male Fischer rats were thus divided into three groups: Group 1 (n = 5) consisted of rats receiving 100 mg kg−1 intra-peritoneal DEN and NMOR at 80 ppm in drinking water ad libitum until 2 weeks prior to sacrifice at 16 weeks; Group 2 (n = 5) also received the same doses of DEN and NMOR until 2 weeks prior to sacrifice, in addition to 20 mg kg−1/day gavaged norfloxacin until the time of sacrifice; Group 3 (n = 6) received no treatment and acted as healthy controls (Table 1). All animals received the same standard non-medicated laboratory rodent chow (Teklad® 4% fat fixed-formula diet from Harlan Laboratories, Blackthorn, United Kingdom). All rats in Groups 1 and 2 developed multifocal HCC, Group 1 displaying a greater extent of liver inflammation than Group 2. All rats in Group 3 had normal liver histology. All animals were sacrificed at 16 weeks, whereupon blood was drawn from the abdominal aorta into heparin containing tubes and livers extracted for histological analysis. All samples were centrifuged and snap-frozen in liquid nitrogen and kept at −80 °C until analysis.
Table 1.
Fischer Rat Groups and Interventions; Groups 1 and 2 Developed HCC.
| Intervention | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| n: | 5 | 5 | 6 |
| Diethylnitrosamine (DEN) | 100 mg kg−1 | 100 mg kg−1 | 0 |
| N-nitrosomorpholine (NMOR) | 80 ppm in water | 80 ppm in water | 0 |
| Norfloxacin | 0 | 20 mg kg−1/day | 0 |
Sample Preparation
Samples were prepared according to standard validated protocols.13 Samples were thawed at room temperature and 200 μL was transferred into 1.5 mL Eppendorf (Eppendorf, Cambridge, UK) tubes to which 400 μL NaCl/D2O (90%/10%) was added. The mixture underwent centrifugation for 5 min at 13,000 rpm and 550 μL of supernatant was transferred to Norell, 5 mm 507-HP-7 NMR tubes (Norell, Landisville, New Jersey, USA) ready for 1H NMR analysis.
Proton Nuclear Magnetic Resonance Spectroscopy
Samples were run in a random non-grouped order under automation on a Bruker Ultrashield Plus™ 600 NMR system operating at 600.22 Hz 1H frequency (Bruker Biospin, Rheinstetten, Germany), fitted with a super-cooled probe head, containing the receiver and radiofrequency coils, cooled to almost 0 K by use of liquid helium (Cryoprobe™, Bruker Biospin, Rheinstetten, Germany). The system was tuned, matched and frequency locked on to 1H as the nucleus of interest. A representative sample was utilised to set shim gradients to ensure a homogenous magnetic field across the sample, a 90° pulse length and the water suppression offset parameters. These settings were saved and utilised for the whole sample set. Pulse programme and acquisition parameters were set according to optimised protocols for blood from the department of Biomolecular Medicine, Imperial College London. Spectra were acquired using Nuclear Overhauser Effect Spectroscopy (NOESY) and Carr-Purcell-Meiboom-Gill (CPMG) sequences at 300 K. One-dimensional (1-D) NOESY pulse sequence was used with water pre-saturation during the relaxation delay (RD) and mixing time (tm), using the following pulse programme: -RD-90°_t-90°-tm-90°-acquire, where RD = 2.0 s and tm = 0.1 s. For each sample, 64 free induction decays (FIDs) were collected into 32,000 data points with a spectral width of 20 ppm. 1H NMR spectra were also acquired using a CPMG-presat sequence to attenuate broad signals from macromolecules, such as protein and lipid.13 For CPMG-presat experiments, the following pulse sequence was used: -RD-90°-(t-180°-t)n-ACQ, where t is the spin echo delay, n is the number of loops and 180° corresponds to a 180° RF pulse. Here, RD = 2 s, t = 0.0002 s, n = 160, acquisition time = 2.73 s and for each sample, 128 FIDs were collected into 32,000 data points with a spectral width of 20 ppm. A line-broadening function of 0.3 Hz was applied prior to Fourier transformation. 1H NMR spectra were manually phased, baseline corrected and referenced to α-glucose at 5.23 ppm in TOPSPIN v2.0 (Bruker Biospin, Rheinstetten, Germany). Spectral peaks were assigned with reference to the literature.14, 15, 16
Spectral Processing
1H NMR spectra were exported to MATLAB R2010 (MathWorks, Natick, Massachusetts, USA), and to avoid influence on analyses from water suppression aberration, the water region from 4.5 to 6 ppm was excluded from all spectra. Data were normalised using median fold-change normalisation to remove the effect of inter-experimental variability. Median spectra for both groups were generated to allow direct visual comparison of average spectra and allow the selection of regions that were visually divergent, in addition to those identified by multivariate analysis, for peak integration. Semi-quantitative metabolite data were obtained and expressed as relative metabolite concentrations, obtained by integrating the total area under the respective metabolite resonances.
Multivariate and Univariate Statistical Analysis
Full resolution data were used for analyses. Data matrices were generated in MATLAB containing ppm variables as columns and sample identities as rows. Matrices were exported to SIMCA-P v12 (Umetrics, Umea, Sweden). After water exclusion, this amounted to a matrix, for both NOESY and CPMG experiments, of 31,181 variables and 16 observations. Data were mean-centred and principal components analysis (PCA) was performed first to model overall variation and identify outliers. Only mean-centred data were used for further analysis. After outliers were identified and excluded, partial least squares discriminant analysis (PLS-DA) was performed to identify the discriminant strength of the metabolite-based model and to generate a loadings plot from which metabolites could be identified, which most greatly contributed to differences between the groups. In SIMCA-P v12, PLS-DA models were generated through sevenfold cross validation. In this method, every 7th sample was excluded (1st, 7th, 14th, 21st and so on), a model generated from the remaining samples and the excluded “training set” predicted back into the model. This was repeated for all the samples (grouping the 2nd, 9th, 16th and 3rd, 10th, 17th and so on) until all the samples had been excluded once. The results were averaged to produce a model, which is externally cross-validated. Spectral peaks, which contributed most to PLS-DA models and those visually different on median spectra comparison, were selected for peak integration. Comparison of integrals was performed using GraphPad™ Prism (La Jolla, California, USA), using Mann–Whitney non-parametric tests of significance, as normal Gaussian distribution could not be assumed, P-values of <0.05 were considered significant.
Results
Fischer Rat Weights
Table 2 records the median weights of the three groups of rats. Healthy controls were 120 g heavier than their liver disease counterparts.
Table 2.
Fischer Rat Weights at the Time of Sacrifice.
| Group 1 (HCC) | Group 2 (HCC + norfloxacin) | Control group | |
|---|---|---|---|
| Median weight (g) | 336.0 | 335.5 | 455.5 |
Liver Size
DEN and NMOR treated livers were pale in colour, bigger in size (approximately 5 cm × 4 cm × 3 cm), and nodular in character with irregular surfaces, showing multiple HCCs on section, which were even paler than the surrounding tissue. In the group treated with norfloxacin in addition to DEN and NMOR, the livers were much brighter, red in colour with smoother outer surfaces, and measured smaller (approximately 4 cm × 3.5 cm × 2 cm). The cut section of the liver in both groups showed multiple HCCs, which were paler than the surrounding tissue. Both HCC groups developed tumours to the same extent with a high tumour burden (Figure 1). In the naive group, all animals had normal bright red, glossy livers with smooth outer surfaces of approximately 3.5 cm × 3 cm × 2 cm in size, and on cut section showing homogenous liver parenchyma devoid of tumour nodules.
Figure 1.
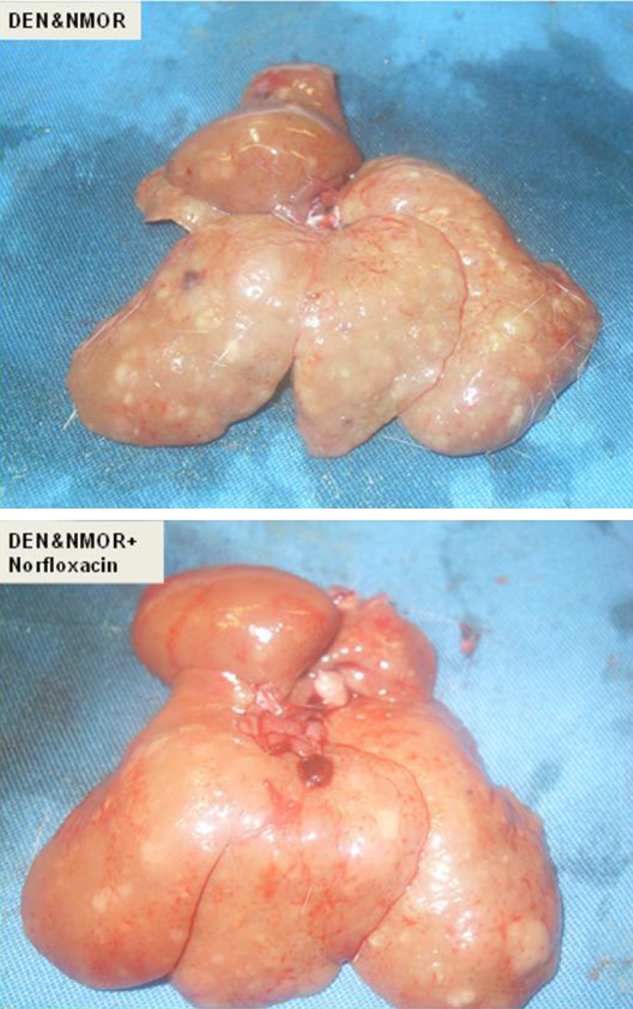
Macroscopic comparison of livers from Group 1 and Group 2. The liver from the rat without norfloxacin treatment is pale with an irregular outer surface and multiple nodules. The liver from the norfloxacin-treated rat is brighter and less nodular in appearance.
Proton Magnetic Resonance Spectral Profiles
The spectral regions displayed high divergence between groups, and these are recorded in Table 3.
Table 3.
Resonance Identities in Rat Plasma Spectra Between 0.5 and 4.5 ppm.
| Label | Chemical shift (ppm) | Multiplicity | Molecule |
|---|---|---|---|
| 1 | 0.84 | Multiplet (broad) | Low-density lipoprotein (CH3) |
| 2 | 0.87 | Multiplet (braod) | Very low-density lipoprotein (CH3) |
| 3 | 1.22 | Multiplet (broad) | Low-density lipoprotein (CH2) |
| 4 | 1.25 | Multiplet (broad) | Very low-density lipoprotein (CH2) |
| 5 | 1.33 | Doublet | Lactate (CH3) |
| 6 | 1.46 | Doublet | Alanine |
| 7 | 1.57 | Multiplet (broad) | Lipid (CH2) |
| 8 | 2.04 | Singlet | Acetyl-glycoprotein |
| 9 | 2.23 | Multiplet (broad) | Lipid (CH2) |
| 10 | 2.69 | Multiplet | Lipid (CH2) |
| 11 | 3.24 | – | Glucose moeities and amino acids |
| 12 | 3.8 | – | Glucose moeties |
| 13 | 4.11 | Quadruplet | Lactate (CH) |
Multivariate Statistical Analysis
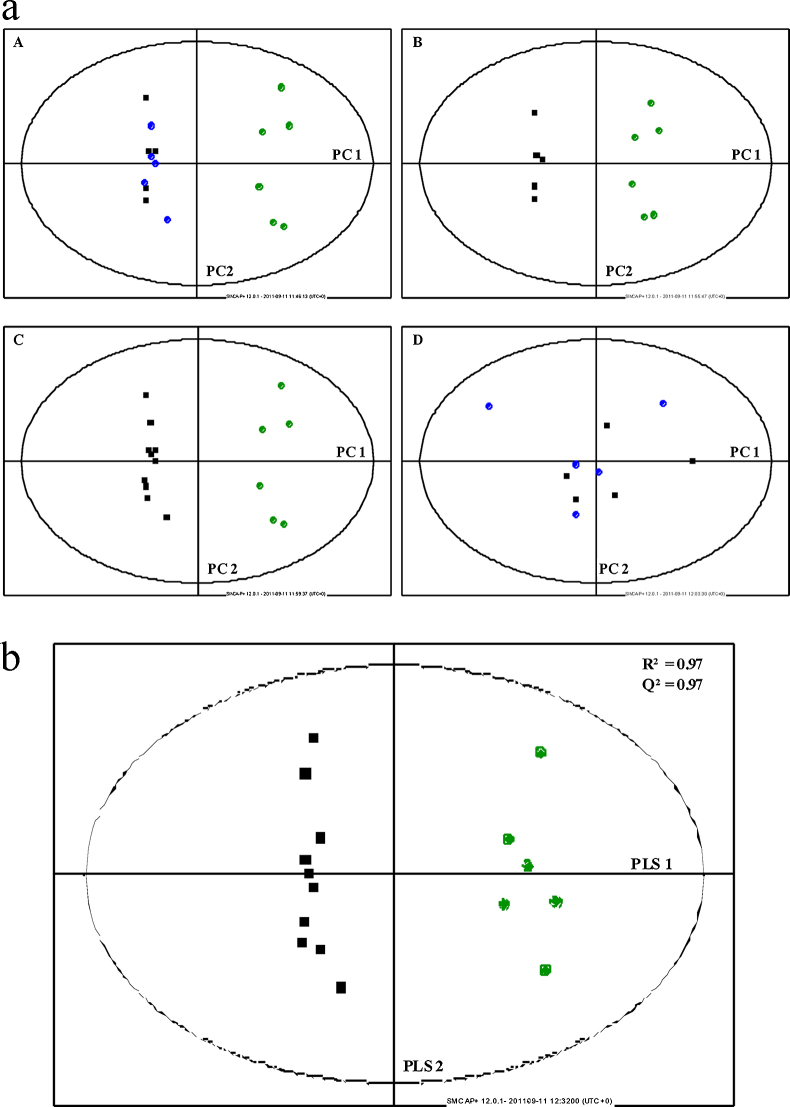
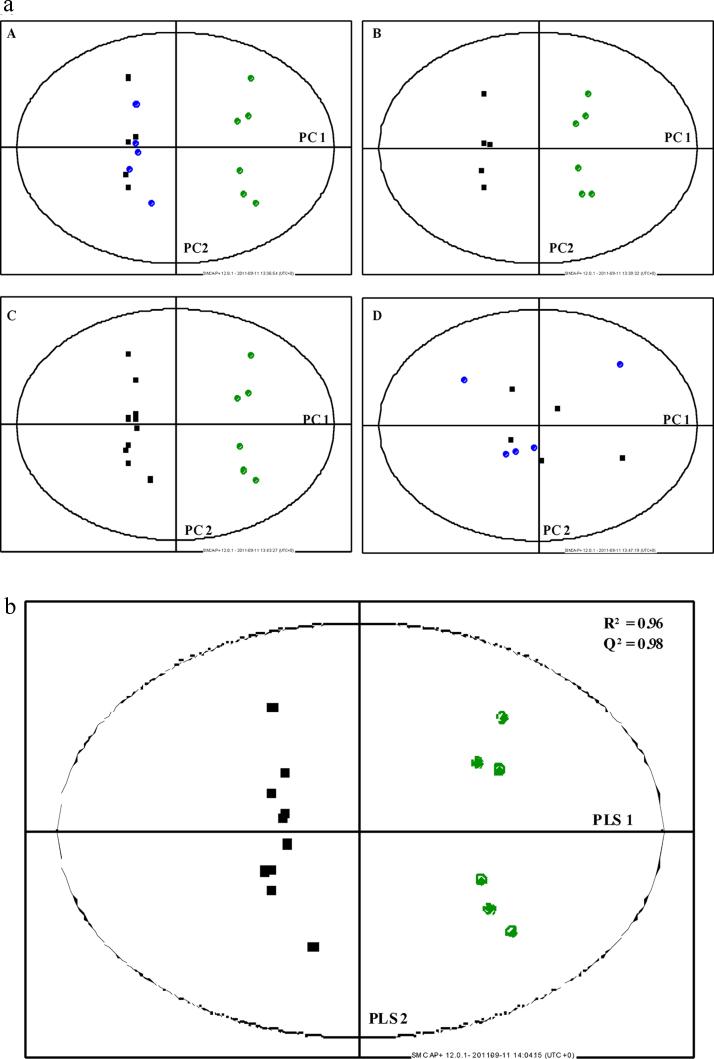
Multivariate analysis scatter plots are displayed in Figure 2, Figure 3, depicting clear clustering with separation of rats with HCC (Groups 1 and 2) and healthy controls. In both NOESY and CPMG studies, Groups 1 (no norfloxacin) and 2 (norfloxacin) were poorly separated by multivariate analysis. Using PLS-DA, data were generated for CPMG and NOESY studies for combined Groups 1 and 2 (those animals with HCC) vs. Group 3 (healthy controls) (Figure 2, Figure 3). The R2 (goodness of fit) and Q2 (goodness of prediction) were greater than 0.95 in both studies. This is reflected in the misclassification matrix and sensitivity and specificity of HCC diagnosis in Table 4, illustrating 100% sensitivity and specificity of HCC diagnosis, for both NOESY and CPMG studies. Loadings plots of PLS-DA components identified the same spectral regions contributing most to multivariate models in both NOESY and CPMG experiments. As a result, only CPMG data were used for subsequent univariate analyses.
Figure 2.
(a) Principal components analysis of rat plasma. Data from NOESY analysis. (A) Groups 1 (■), 2 (●) and 3 (●); (B) Group 1 and Group 3; (C) Groups 1 and 2 combined and Group 3; (D) Groups 1 and 2. PC = Principal Component. (b) Partial least squared discriminant analysis of rat plasma. Data from NOESY analysis. HCC + norfloxacin (■) and healthy controls (●).
Figure 3.
(a) Principal components analysis of rat plasma. Data from CPMG analysis. (A) HCC (■), HCC + norfloxacin (●) and healthy controls (●); PC = Principal Component. (b). Partial least squared discriminant analysis of rat plasma. Data from CPMG analysis. HCC + norfloxacin (■) and healthy controls (●).
Table 4.
Misclassification Matrix for PLS-DA Prediction of Disease.
| Predicted as HCC | Predicted as no HCC | Total | Sensitivity/Specificity | |
|---|---|---|---|---|
| HCC | 10 | 0 | 10 | 100%/100% |
| No HCC | 0 | 6 | 6 |
Univariate Statistical Analysis
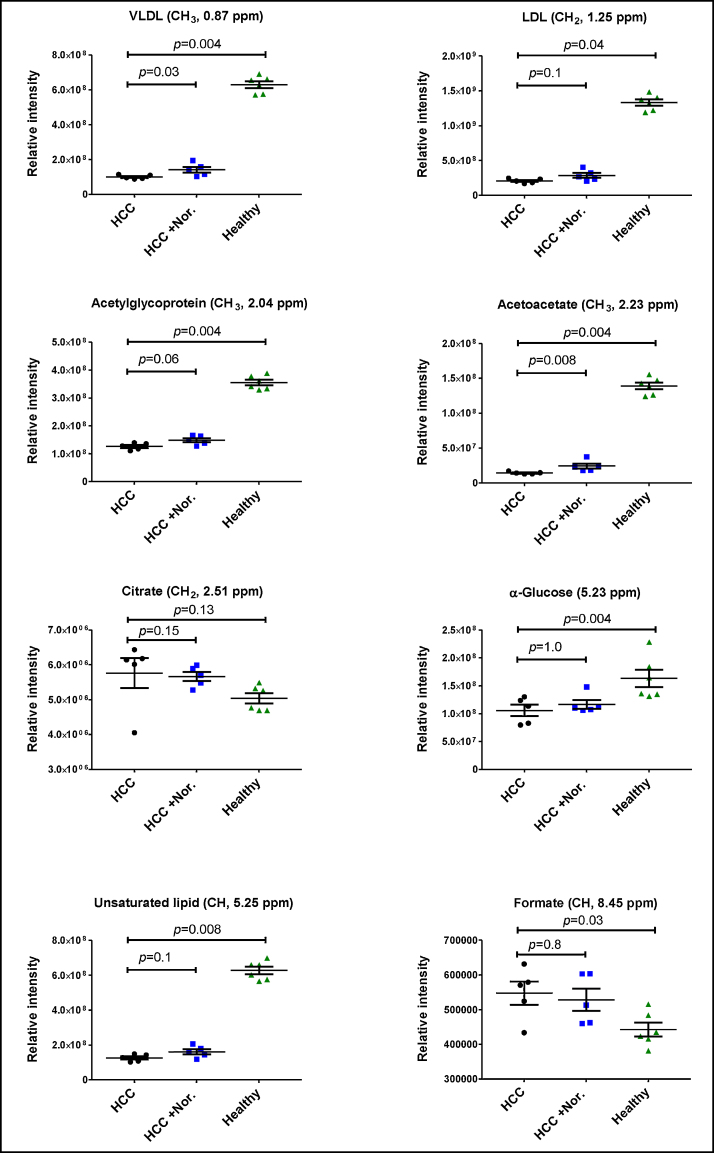
Variables from CPMG PLS-DA models that contributed most to variation between those animals with (Groups 1 and 2) and those without HCC (Group 3) are tabulated and graphically illustrated (Table 5; Figure 4). Plasma LDL, VLDL, acetyl-glycoprotein, α-glucose and unsaturated fatty acids were all significantly reduced in rats with HCC (P ≤ 0.001). Plasma citrate and formate were significantly increased in animals with HCC (P = 0.02).
Table 5.
Median Metabolite Levels in Healthy and Diseased Animals.
| Chemical shift (ppm) | Molecule | HCC | HCC + Nor. | Controls | P-value |
|---|---|---|---|---|---|
| 0.80–0.85 | LDL (CH3) | 1.70 × 108 | 1.48 × 108 | 1.67 × 108 | 0.55†, 0.60‡ |
| 0.86–0.89 | VLDL (CH3) | 9.58 × 107 | 1.37 × 108 | 6.41 × 108 | 0.03†, 0.004‡ |
| 1.24–1.28 | LDL (CH2) | 2.05 × 108 | 2.66 × 108 | 1.35 × 109 | 0.10†, 0.004‡ |
| 1.28–1.31 | VLDL (CH2) | 1.48 × 108 | 2.60 × 108 | 1.09 × 109 | 0.03†, 0.004‡ |
| 2.03–2.06 | Acetyl-glycoprotein | 1.41 × 107 | 2.27 × 107 | 3.51 × 108 | 0.06†, 0.004‡ |
| 2.50–2.52 | Citrate | 6.14 × 108 | 5.69 × 106 | 5.01 × 106 | 0.15†, 0.13‡ |
| 2.22–2.24 | Acetoacetate | 1.41 × 107 | 2.27 × 107 | 1.41 × 108 | 0.008†, 0.004‡ |
| 5.22–5.24 | α-glucose | 1.27 × 108 | 1.59 × 108 | 1.50 × 108 | 1.0†, 0.004‡ |
| 5.25–5.38 | Unsaturated lipid | 1.59 × 108 | 6.32 × 108 | 6.32 × 108 | 0.1†, 0.008‡ |
| 8.44–8.45 | Formate | 570,230 | 512,847 | 429,393 | 0.8†, 0.03‡ |
Key: Nor = norfloxacin; LDL = low-density lipoprotein; VLDL = very low-density lipoprotein. Relative metabolite concentrations obtained by integrating total area under the respective metabolite resonances.
HCC group vs. HCC + norfloxacin.
HCC group vs. Controls.
P-values calculated using Mann–Whitney non-parametric tests of significance.
Figure 4.
Relative intensities (integral values) of selected metabolites.
Discussion
The aims of this study were to investigate the plasma 1H NMR metabolite changes that occur in the presence of HCC. The effect of norfloxacin upon these profiles was also evaluated, as gut sterilisation has been shown to reduce circulating endotoxin levels and hepatic inflammation.8, 9, 10, 11, 12 The metabolite profiles of those animals with HCC differed greatly from healthy control animals, mainly with respect to lipid lipoprotein associated species, displaying clear separation on PCA plots and excellent classification by PLS-DA. The animals with HCC, however, were over 100 g lighter than healthy controls, and this is clearly a significant factor, which influences interpretation of the findings. It is not clear, however, if this weight difference explains the specific alteration in metabolites. By contrast, it should be noted that there was a significant 2-week washout period of both DEN and NMOR prior to sacrifice and given the rapid metabolism of these agents, it is unlikely that these agents had a significant, direct contributory effect on the metabolic profiles of the animals with HCC, given the high tumour burden in these animals.
Low-density lipoprotein, VLDL and unsaturated fatty acids were significantly reduced in animals with tumours. There are several possible explanations. First, the absorption of fat, via chylomicrons, may be reduced, reducing the provision of lipid for the production of VLDL by the liver, although this would be unlikely as vastly reduced absorption would have been required to cause the results seen. Second, the findings may indicate cancer cachexia through an increased basal metabolic rate, which is defined as loss of skeletal muscle mass, with or without loss of body fat.17 Cancer cachexia is a complex condition likely to be cytokine mediated and indicative of end-stage disease.17 There are conflicting findings that support and argue against this: first, there is little evidence of increased muscle breakdown in rats with HCC, with no rise in plasma creatine or creatinine. Second, if fat were being actively metabolised in normal tissue, it would be mostly through β-oxidation in mitochondria, a by-product of which is acetoacetate. Acetoacetate plasma levels were significantly reduced in animals with HCC, which, on the face of it, could indicate reduced fatty acid metabolism, not in keeping with the use of fatty acids as an energy substrate. Ketoacids, however, are not definitive markers of fat metabolism, but they can be oxidised in cancer cells through ω-oxidation as an energy source, a hallmark of cancer cachexia. It may, therefore, be that fat is being mobilised for energy production through this alternative pathway in these animals with higher basal metabolic rates.
These results may also indicate deteriorating liver function. In addition to its multiple roles of coagulation cascade component, bile acid and vitamin production, the liver produces VLDL particles, which are then circulated in the blood providing tissues with fatty acid substrates. Reduced production of VLDL particles results in reduced production of LDL and HDL particles, as observed here. It may be that the functional status of the liver, induced by tumour burden and inflammation caused by the carcinogens, DEN and NMOR, is reduced. It is also possible that the lipids are being used to fuel cellular division, providing the substrate for cell membranes in rapidly dividing tumours. This would then account for the reduced by-products of fatty oxidation that we observed. Finally, DEN is a potent carcinogen and its effect on lipid metabolism, aside from effects on liver inflammation, is not known. Animal studies have shown that DEN induces lipid peroxidation, which may be a factor in interpreting the results presented here.18 The effects of NMOR on lipid metabolism are not known. However, given the washout period prior to sacrifice, the direct effects of DEN and NMOR are likely to be small.4
On the positive side, our findings presented here do parallel those of a recent study undertaken by Wang and colleagues, where male Sprague-Dawley rat liver tumour homogenate, induced by DEN, were analysed using 1H NMR spectroscopy.4 A raised level of LDL, VLDL and choline was observed in tumours, suggesting their increased incorporation into the tumour mass. Furthermore, Tesiram and colleagues observed substantial increases in glycerol backbone-containing phospholipids in choline-deficient Fischer rats with hepatic adenomas.3 In this study, diseased rats were lighter than healthy controls, but the growth characteristics of both groups were similar, suggesting an alteration in fat storage, rather than muscle development.
Acetylglycoproteins are known to be produced by hepatocytes and released in response to stressful stimuli and can be induced by a number of different inflammatory conditions, including cancer.19, 20 Acetylglycoproteins were significantly reduced in animals with HCC, which could also imply a decreased functional status of hepatocytes due to disease. The macroscopic appearance of the rat livers, almost completely infiltrated with multi-nodular tumour, would support the theory that less functional tissue remains to produce hepatic proteins.
The effect of norfloxacin on plasma NMR metabolic changes was modest, with phenotypes (all DEN/NMOR + norfloxacin treated animals also developed HCC) and metabolic profiles more similar to DEN/NMOR animals than controls. There is no presentable evidence that this slight alteration is due to reduced bacterial translocation and liver inflammatory status in this study. Furthermore, DEN is dependent upon the action of cytochrome P450 enzymes for conversion to its active metabolites, and norfloxacin has been shown to suppress the activities of these enzymes.21, 22 The results observed in the norfloxacin-treated group may, therefore, be due to enzyme inhibition by norfloxacin rather than alterations in bacterial translocation.
In conclusion, the spectral profiles of plasma in rats with HCC display marked changes with relation to lipid metabolism and cellular turnover. Norfloxacin appears to abrogate these effects slightly, possibly through cytochrome P450 enzyme inhibition, but an effect on the microbiome cannot be excluded. The results of this study have provided an insight to the altered hepatic lipid metabolism in the presence of HCC and corroborate previous work.3, 4 Interpretation must be cautious, given the small sample number. The 1H NMR changes in lipid profiles are likely due to a combination of cancer cachexia and reduced liver function, although certain corroboratory findings, such as an increase in ketoacids, are missing. Further studies are required to characterise lipid subspecies. Low-density lipoprotein, VLDL and unsaturated fatty acids cover a broad range of lipid subspecies, and it would be important to identify whether any particular species is associated with HCC. For this, a more sensitive method of lipid analysis is required, such as liquid chromatography mass spectrometry. Similar studies also need to be undertaken in the human population to test the translatability of findings. There is evidence to show that lipid metabolism is also altered in human cases of HCC, suggesting a good association between rodent and human models of the disease, enhancing the clinical relevance of rodent models.23, 24, 25, 26, 27, 28, 29, 30
Conflicts of interest
The authors have none to declare.
Acknowledgements
All authors acknowledge the support of the National Institute for Health Research Biomedical Research Centre at Imperial College London for infrastructure support. MIFS was supported by grants from the Trustees of the London Clinic, London. MMEC is supported by a Fellowship from the Sir Halley Stewart Trust (Cambridge, United Kingdom). MMEC and SDT-R hold grants from the United Kingdom Medical Research Council.
References
- 1.Gao H., Dong B., Liu X., Xuan H., Huang Y., Lin D. Metabonomic profiling of renal cell carcinoma: high-resolution proton nuclear magnetic resonance spectroscopy of human serum with multivariate data analysis. Anal Chim Acta. 2008;624(2):269–277. doi: 10.1016/j.aca.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 2.Yin P., Wan D., Zhao C. A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Mol Biosyst. 2009;5(8):868–876. doi: 10.1039/b820224a. [DOI] [PubMed] [Google Scholar]
- 3.Tesiram Y.A., Saunders D., Towner R.A. Application of proton NMR spectroscopy in the study of lipid metabolites in a rat hepatocarcinogenesis model. Biochim Biophys Acta. 2005;1737(1):61–68. doi: 10.1016/j.bbalip.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Zhang S., Li Z. (1)H-NMR-based metabolomics of tumor tissue for the metabolic characterization of rat hepatocellular carcinoma formation and metastasis. Tumour Biol. 2011;32(1):223–231. doi: 10.1007/s13277-010-0116-7. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez J., Navasa M., Planas R. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133(3):818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Hurtado I., Zapater P., Bellot P. Interleukin-10-mediated heme oxygenase 1-induced underlying mechanism in inflammatory down-regulation by norfloxacin in cirrhosis. Hepatology. 2011;53(3):935–944. doi: 10.1002/hep.24102. [DOI] [PubMed] [Google Scholar]
- 7.Toffanin S., Cornella H., Harrington A., Llovet J. HCC is promoted by bacterial translocation and TLR-4 signaling: a new paradigm for chemoprevention and management. Hepatology. 2012;56(5):1998–2000. doi: 10.1002/hep.26080. [DOI] [PubMed] [Google Scholar]
- 8.Gines P., Rimola A., Planas R. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology (Baltimore, MD) 1990;12(4 Pt 1):716–724. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 9.Llovet J.M., Bartoli R., Planas R. Selective intestinal decontamination with norfloxacin reduces bacterial translocation in ascitic cirrhotic rats exposed to hemorrhagic shock. Hepatology (Baltimore, MD) 1996;23(4):781–787. doi: 10.1002/hep.510230419. [DOI] [PubMed] [Google Scholar]
- 10.Frances R., Zapater P., Gonzalez-Navajas J.M. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology (Baltimore, MD) 2008;47(3):978–985. doi: 10.1002/hep.22083. [DOI] [PubMed] [Google Scholar]
- 11.Rabiller A., Nunes H., Lebrec D. Prevention of gram-negative translocation reduces the severity of hepatopulmonary syndrome. Am J Respir Crit Care Med. 2002;166(4):514–517. doi: 10.1164/rccm.200201-027OC. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Gu Y., Chen Y. Intestinal flora imbalance results in altered bacterial translocation and liver function in rats with experimental cirrhosis. Eur J Gastroenterol Hepatol. 2010;22(12):1481–1486. doi: 10.1097/MEG.0b013e32833eb8b0. [DOI] [PubMed] [Google Scholar]
- 13.Beckonert O., Keun H.C., Ebbels T.M. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2(11):2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 14.Holmes E., Loo R.L., Stamler J. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453(7193):396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholson J.K., Foxall P.J., Spraul M., Farrant R.D., Lindon J.C. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995;67(5):793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- 16.Wishart D.S., Tzur D., Knox C. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35(Database issue):D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisdale M.J. Cancer cachexia. Curr Opin Gastroenterol. 2010;26(2):146–151. doi: 10.1097/MOG.0b013e3283347e77. [DOI] [PubMed] [Google Scholar]
- 18.Jayakumar S., Madankumar A., Asokkumar S. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012;360(1):51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Holmes E., Tang H. Experimental metabonomic model of dietary variation and stress interactions. J Proteome Res. 2006;5(7):1535–1542. doi: 10.1021/pr0504182. [DOI] [PubMed] [Google Scholar]
- 20.Baumann H., Jahreis G.P., Gaines K.C. Synthesis and regulation of acute phase plasma proteins in primary cultures of mouse hepatocytes. J Cell Biol. 1983;97(3):866–876. doi: 10.1083/jcb.97.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salcido-Neyoy M.E., Sierra-Santoyo A., Beltran-Ramirez O., Macias-Perez J.R., Villa-Trevino S. Celecoxib enhances the detoxification of diethylnitrosamine in rat liver cancer. World J Gastroenterol. 2009;15(19):2345–2350. doi: 10.3748/wjg.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLellan R.A., Drobitch R.K., Monshouwer M., Renton K.W. Fluoroquinolone antibiotics inhibit cytochrome P450-mediated microsomal drug metabolism in rat and human. Drug Metab Dispos. 1996;24(10):1134–1138. [PubMed] [Google Scholar]
- 23.Budhu A., Roessler S., Zhao X. Integrated metabolite and gene expression profiles identify lipid biomarkers associated with progression of hepatocellular carcinoma and patient outcomes. Gastroenterology. 2013;144(5):1066–10750. doi: 10.1053/j.gastro.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S., Yin P., Zhao X. Serum lipid profiling of patients with chronic hepatitis B, cirrhosis, and hepatocellular carcinoma by ultra fast LC/IT-TOF MS. Electrophoresis. 2013;34(19):2848–2856. [PubMed] [Google Scholar]
- 25.Fitian A.I., Nelson D.R., Liu C., Xu Y., Ararat M., Cabrera R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 2014;34(9):1428–1444. doi: 10.1111/liv.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min H.K., Sookoian S., Pirola C.J., Cheng J., Mirshahi F., Sanyal A.J. Metabolic profiling reveals that PNPLA3 induces widespread effects on metabolism beyond triacylglycerol remodeling in Huh-7 hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2014;307(1):G66–G76. doi: 10.1152/ajpgi.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson A.D., Maurhofer O., Beyoglu D. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011;71(21):6590–6600. doi: 10.1158/0008-5472.CAN-11-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J.M., Skill N.J., Maluccio M.A. Evidence of aberrant lipid metabolism in hepatitis C and hepatocellular carcinoma. HPB. 2010;12(9):625–636. doi: 10.1111/j.1477-2574.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita T., Honda M., Takatori H. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol. 2009;50(1):100–110. doi: 10.1016/j.jhep.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L., Wang Q., Yin P. Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Anal Bioanal Chem. 2012;403(1):203–213. doi: 10.1007/s00216-012-5782-4. [DOI] [PubMed] [Google Scholar]