Abstract
The distinction between breast cancer and benign breast diseases with nipple discharge remains an important diagnostic challenge. The purpose of this study was to predict the potential usefulness of tumor markers in nipple discharge and to investigate the relationship of tumor markers and clinical characteristics with breast cancer.One hundred and eleven patients with nipple discharge received breast surgery from November 2013 to December 2014 were included in the study. We evaluated levels of five tumor markers (CEA, CA153, CA199, CA724 and AFP) prior to treatment. Patients were divided into two groups according to postoperative pathological results: 30 cases in breast cancer group and 81 cases in benign group. The relationships of clinical characteristics with breast cancer were investigated by multivariate analysis with a logistic regression model.It showed significant differences in levels of nipple discharge CEA (P < 0.001) and CA153 (P = 0.014), but not CA199 (P = 0.856), CA724 (P = 0.171), AFP (P = 0.834) among two groups. Logistic regression analysis demonstrated complaint, age, menopause, abnormal palpable mass, CEA and CA153 were associated with breast cancer. In summary, measurements of CA199, CA724 and AFP in nipple discharge are not of great clinical value. Detecting CEA and CA153 in nipple dischargecould potentially be used for the early detection of breast cancer with in high-risk populations.
Keywords: Breast cancer, diagnosis, nipple discharge, CA199, CA724, AFP
Introduction
Besides breast mass and breast pain, nipple discharge is also a relatively common breast complaint accounting for up to 5% of which women seek medical advice [1]. Of the patients presented with nipple discharge, 10-20% would have underlying malignancy [2,3].
An effective diagnostic tool is needed for accurate stratification of nipple discharge patients for further work up and treatment. Levels of tumor markers in nipple discharge may help to establish the diagnosis of different breast diseases. Tumor markers, such as carcinoembryonic antigen (CEA), cancer antigen 153 (CA153), cancer antigen 199 (CA199), cancer antigen 724 (CA724) and alpha-fetoprotein (AFP) are all glycoproteins with altered glycan profiles in cancer progression. CEA is overexpressed in the majority of colon cancers, half of all breast cancers and nonsmall cell lung cancers [4-6]. CA153 is a transmembrane glycoprotein up-regulated in carcinomas of epithelial origin, including breast cancer, ovarian cancer, pancreatic cancer, and multiple myeloma [7]. CA153 has been shown to be an independent predictor of cancer recurrence as well as a powerful prognostic indicator for patients at advanced-stage breast cancer [8]. In our previous study, preoperative CEA, CA153 independently indicated reliability and predictability in breast cancer with nipple discharge. Patients with CEA > 224.3 ng/mL and CA153 > 1368.2 U/mL generally had a breast cancer pathological finding.It was clarified thatCA199 and AFP had increased serum levels in human breast cancer cases [9,10]. AFP was considered as a breast cancer risk factor [11,12]. CA724 was expressed in colorectal, gastric cancer and ovarian cancer [13]. However, CA724, CEA and CA199 combination was considerable to improve sensitivity without impairing specificity [13]. Bian also indicated that combined assay of CA724 and CA153 can provide diagnosis value for ovarian cancer [14,15]. According to previous studies, combination of CA724 and other tumor markers may improve diagnosis for clinical use.
In this study, we determined levels of five tumor markers in nipple discharge and verify CEA and CA153 diagnostic values in differentiation of breast cancer and benign breast diseases. The present study was also aimed to find out the potential useful tumor markers including CA199, CA724 and AFP to use as supplementary test for the diagnosis of breast cancer. At the same time, we evaluated the relationship between tumor markers, other clinical characteristics like age, menopausal status in women with nipple discharge and breast cancer.
Materials and methods
Subject
A total of 111 patients with complaint of nipple discharge were scheduled to have surgeries were included in our study between November 2013 and December 2014 in Qilu Hospital of Shandong University. Informed consents were obtained from all participants. This study was approved by ethics committee of Qilu Hospital of Shandong University. The study cohort included women with unilateral nipple discharge. Patients had received no preoperative treatment. They were divided into two groups (breast cancer group and benign group) according to the postoperative pathological diagnosis. 111 patients were pathologically confirmed postoperatively with breast cancer, including the following histological subtypes: invasive ductal carcinoma (n = 11), intraductal papillary carcinoma (n = 11), ductal carcinoma in situ (n = 8). 30 patients were diagnosed with benign breast diseases including intraductal papilloma (n = 57), mammary duct ectasia (n = 19), breast fibroadenoma (n = 5) (Figure 1).
Figure 1.
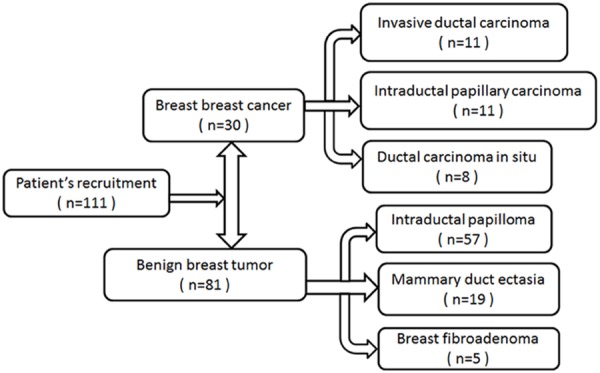
Patientsrecruitment.
Nipple discharge collection and laboratory methods
All samples were collected before any treatment was initiated within 2 days after hospitalization. Nipple was cleansed first with alcohol swabs to remove cellular debris. Nipple discharge was expressed by manual compression of the breast. No serious complications occurred. Droplet of nipple discharge was collected in an eppendorf tube. The tube was then stored in dedicated refrigerator at 4°C. The quantity of collected nipple discharge varied from 20 μL to 200 μL. Samples were transported to the laboratory department within 8 hours after collection. Viscous samples were diluted up to 20-fold with normal saline before centrifugation and storage at 4°C. Concentrations of CEA, CA153, CA199, CA724 and AFP in nipple discharge were quantitativelymeasured via an automated test system utilizing sandwich electro chemiluminescence immunoassay (ECLIA) assay kits (Roche cobas e601 analyzer, Roche Diagnostics). All tumor markers assays were performed at Qilu Hospital of Shandong University according to manufacturer’s protocol. The laboratory personnel were blinded to the clinical information. Commercial reference control sera were used for quality control and calibration.
Statistical analysis
Data of tumor markers were expressed as mean ± standard error of the mean (SEM). Comparisons of data between two groups were performed using Kruskal-Wallis test or Mann-Whitney U test. Receiver operating characteristic (ROC) analysis was used to identify the cutoff values of CEA and CA153. Samples from patients with histologic-proven breast malignancies were used in determining cutoff values with max-sum of sensitivity and specificity. Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) were calculated based on the cutoff value for CEA and CA153. Patients’ clinical characteristics were summarized descriptively. Detailed clinical characteristics of patients including complaint, side of nipple discharge, age, menarche, menopause, average menstrual cycle, age at first child, number of child, lactation, Interval between menarche and primiparity, family history, mammary gland disease history, body mass index and palpable masswere obtained to build electronic databases. The Pearson Chi-square or Fisher’s exact test was used to evaluate these qualitative data. Logistic regression analysis was used to select the risk factors for breast cancer probability. Variables that achieved statistical significance in univariate analysis were included subsequently in a multivariate analysis. All P values were two-sided, P < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS 17.0 software.
Results
There are 111 patients enrolled in the study hospitalized for breast surgery. The patients are aged between 20 and 72 years old (average = 44.7). It showed that invasive ductal carcinoma (n = 11, 36.7%) and intraductal papillary carcinoma (n = 11, 36.7%) were the main cause of breast cancer with nipple discharge, followed by ductal carcinoma in situ (n = 8, 26.7%). It showed that intraductal papilloma (n = 57, 70.4%) was the main cause of benign diseases with nipple discharge (Figure 1). The clinical characteristics of the patients are summarized in Table 1. The mean ages of the two groups were 48.4 years (range, 28-72 years) in breast cancer group and 43.3 years (range, 20-64 years) in benign group, respectively, and statistic significance differences can be found (P = 0.002). Complaints, menopausal age, palpable mass in preoperative examination were compared between the two groups, statistic significant differences (P < 0.05) were observed. There was no statistical difference (P > 0.05) between two groups in term of general clinical data, such as side of nipple discharge, menarche, age at first child, etc.
Table 1.
Logistic regression results for the relationship between breast cancer and benign breast diseases
| Variable | Subgroup | Breast cancer | Benign breast diseases | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total no. | Total no. | OR (95% CI.) | P | OR (95% CI.) | P | ||
| Complaint | 3.66 (1.45-9.22) | 0.006** | 0.66 (0.17-2.65) | 0.561 | |||
| Nipple discharge | 17 | 67 | |||||
| Mass with nipple discharge | 13 | 14 | |||||
| Side of nipple discharge | 0.125 | ||||||
| Left | 15 | 25 | 2.05 (0.52-8.17) | 0.307 | |||
| Right | 12 | 37 | 0.54 (0.22-1.35) | 0.187 | |||
| Both | 3 | 19 | |||||
| Age | 4.19 (1.67-10.51) | 0.002** | 12.68 (1.11-144.47) | 0.041* | |||
| < 50 | 16 | 67 | |||||
| ≥ 50 | 14 | 14 | |||||
| Menarche | 1.06 (0.45-2.46) | 0.898 | |||||
| ≤ 14 | 17 | 47 | |||||
| > 14 | 13 | 34 | |||||
| Menopause | 4.87 (1.86-12.74) | 0.001** | 0.87 (0.09-8.65) | 0.903 | |||
| Premenopausal | 17 | 70 | |||||
| Postmenopausal | 13 | 11 | |||||
| Average menstrual cycle | 0.87 (0.38-2.02) | 0.745 | |||||
| ≤ 28 | 16 | 46 | |||||
| > 28 | 14 | 35 | |||||
| Age at first child | 0.587 | ||||||
| ≤ 26 | 13 | 44 | 1.45 (0.33-6.42) | 0.624 | |||
| ≤ 30 | 3 | 7 | 0.92 (0.21-4.09) | 0.911 | |||
| > 30 | 14 | 30 | |||||
| Number of child | 2.74 (0.32-23.29) | 0.355 | |||||
| Nullipara | 1 | 7 | |||||
| Multipara | 29 | 74 | |||||
| Lactation | 1.53 (0.31-7.67) | 0.602 | |||||
| No | 2 | 8 | |||||
| Yes | 28 | 73 | |||||
| Interval between menarche and primiparity | 1.14 (0.41-3.22) | 0.801 | |||||
| < 10 | 24 | 63 | |||||
| ≥ 10 | 6 | 18 | |||||
| Family history | 0.96 (0.31-2.93) | 0.94 | |||||
| Yes | 5 | 14 | |||||
| No | 25 | 67 | |||||
| Mammary gland disease history | 0.25 (0.03-2.00) | 0.189 | |||||
| Yes | 1 | 10 | |||||
| No | 29 | 71 | |||||
| BMI | 0.497 | ||||||
| < 25 | 7 | 27 | 2.41 (0.14-40.26) | 0.541 | |||
| < 30 | 22 | 53 | 3.86 (0.21-69.67) | 0.361 | |||
| ≥ 30 | 1 | 1 | |||||
| Palpable mass | 4.1 (1.70-9.92) | 0.002** | 5.72 (1.48-22.18) | 0.012* | |||
| No | 11 | 57 | |||||
| Yes | 19 | 24 | |||||
| CEA level | 6.60 (2.63-16.57) | < 0.001** | 8.51 (2.60-27.86) | < 0.001** | |||
| < 224.3 ng/ml | 12 | 66 | |||||
| ≥ 224.3 ng/ml | 18 | 15 | |||||
| CA153 level | 2.88 (1.17-7.07) | 0.021* | 4.88 (1.37-17.36) | 0.015* | |||
| < 1368.2 U/ml | 17 | 64 | |||||
| ≥ 1368.2 U/ml | 13 | 17 | |||||
Complaint, age, menopause, palpable mass, CEA level and CA153 level showed significant differences between the two groups. Abbreviation: 95% CI, 95% confidence interval; BMI, Body index.
P < 0.05;
P < 0.01.
The levels of CEA, CA153, CA199, CA724 and AFP in nipple discharge of patients in two groups are presented in Figure 2. Mann-Whitney U test for differences between the studied groups in nipple discharge tumor markers were performed. The levels of CEA and CA153 in the breast cancer group were significantly higher than those of the benign group (P < 0.05). The capacity of CEA and CA153 to differentiate causes of nipple discharge was assessed with cutoff values made by previous study. Evaluation of ROC analysis based on sensitivity and specificity established cut-off values for CEA and CA153 (Figure 3). The cut-off value of CEA was 224.3 ng/ml and CA153 was 1368.2 U/ml, respectively. Levels of the other three tumor markers (CA199, CA724 and AFP) newly added in the study had no statistical difference between two groups (P > 0.05).
Figure 2.
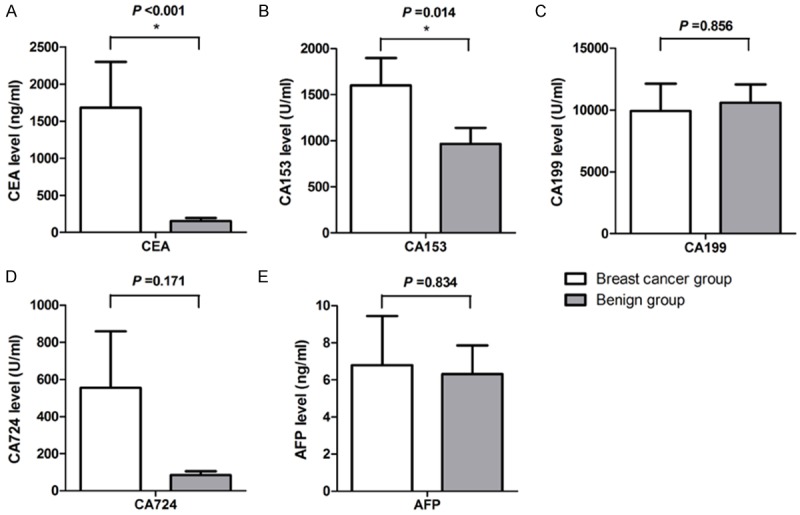
CEA, CA153, CA199, CA724 and AFP levels in nipple discharge: showed statistically higher concentrations of CEA (A) and CA153 (B) in breast cancer group compared to benign group (P < 0.001, P = 0.014, respectively). No statistically significant difference in levels of CA199 (C), CA724 (D) and AFP (E) (P > 0.05) between two groups.
Figure 3.
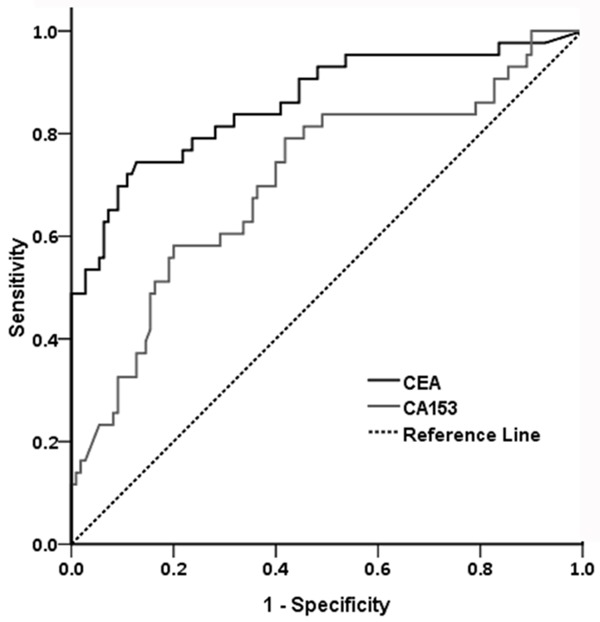
Receiver operating characteristic (ROC) analysis for the diagnosis of CEA and CA153 in nipple discharge. According to the analysis in previous study (cancerous breasts = 43, benign controls = 110), cut-off values of CEA and CA153 were assessed (CEA was 224.3 ng/ml and CA153 was 1368.2 U/ml, respectively).
When used to analyze breast cancer group versus benign group, the sensitivities, specificities, positive predictive value (PPV) and negative predictive value (NPV) of CEA and CA153 at their respective cut-off values are outlined in Table 2. In this situation, we calculated that sensitivity was 60.0%, specificity was 81.48%, positive predictive value was 54.55%, negative predictive value 84.62%, and accuracy was 75.68% for CEA which performed better than CA153.
Table 2.
Diagnostic performance of tumor marker concentrations
| Tumor makers | Sensitivity | Specificity | PPV | NPV | Accuracy | Cutoff value |
|---|---|---|---|---|---|---|
| CEA | 60.00 (18/30) | 81.48 (66/81) | 54.55 (18/33) | 84.62 (66/78) | 75.68 (84/111) | 224.3 |
| CA153 | 43.33 (13/30) | 80.00 (64/80) | 44.83 (13/29) | 79.01 (64/81) | 70.00 (77/110) | 1368.2 |
Note: Data are percentages, with numbers used to calculate them in parentheses. NPV = negative predictive value, PPV = positive predictive value.
In Table 1, breast cancer were more likely in older patients (age > 50) than younger woman (P = 0.001). It was observed that patients with the complaint of masses with nipple discharge would probably be diagnosed as breast cancer (P = 0.006). Postmenopausal women were significantly different from premenopausal women in the breast cancer group (P = 0.001). In addition, patients with breast cancer showed a significant correlation with palpable mass in physical examination (P = 0.002). Combining with significant values of CEA and CA153, a multiple logistic regression analysis revealed that breast cancer was significantly related to four factors: age, palpable mass, CEA level and CA153 level. These four factors were used as a dependent variable associated with breast cancer.
Discussion
Currently, there are few studies on nipple discharge tumor markers in detection of breast cancer.In our previous study, the identification of CEA and CA153 for detection of breast cancer with nipple discharge has produced given definite indications for their possible use in clinical practice. In this study, we investigated CA199, CA724 and AFP as potential tumor markers and validated CEA and CA153 to differentiate breast cancer from benign diseases.
The presence of tumor markers, such as CEA and CA199 is closely associated with invasion and metastasis of many malignancies [16]. CA199 is of great clinical value for the diagnosis and prognosis of pancreatic cancer [17], and also had been observed in gastrointestinal cancer [18-20]. Other potential markers are CA724, known for colorectal and gastric cancer, ovarian cancer [21,22], AFP also known for ovary, testis and liver cancer [23]. A previous study indicated that AFP in serum suppressed estrogen-dependent growth of breast cancer cells [24]. In other two studies, it reported protective associations of maternal breast cancer with increasing circulating levels of AFP, particularly at younger ages [25,26]. Unfortunately, there is paucity of article showed the level of CA199, CA724 and AFP had relationship to breast cancer with nipple discharge in the past.
Our results showed that the levels of CEA and CA153 in breast cancer group were significantly higher than those of the control group (P < 0.05). It obtained coincide with the previous experimental results. It indicated that CEA and CA153 can play an important role in the process of diagnosis in breast cancer with nipple discharge. The present study was the first time to predict the potential usefulness of CA199, CA724 and AFP in nipple discharge. According to data, mean levels of CA724 in cancer group were higher than benign control but showed no difference between two groups. Nonetheless, CA199 and AFP in cancer group had no statistical significance when compared with benign group. Measurements of CA199, CA724 and AFP in nipple discharge were not of great clinical value for assessment to the clinician.
This study evaluated the correlations of significant tumor markers and patient clinical characteristics with postoperative pathological analysis. Moreover, Chi-square test showed complaint, age, menopause status, palpable mass in physical examination, CEA and CA153 were all strongly associated with breast cancer. These results showed that above factors were correlated with the occurrence and development of breast cancer. The patients with breast cancer exhibited significantly higher CEA and CA153 than those with benign breast diseases. We also showed that patients in cancer group were significantly older than controls. Masses in complaints or physical examination and postmenopausal status occurred more frequently in breast cancer patients. Nonetheless, themultivariate logistic regression analysis revealed age, palpable mass in physical examination, CEA and CA153 showed independent influential factors for breast cancer, differences between complaint and menopause status did not reach statistical significance.
When it comes to the limitations of this study, because the cases only came from the Department of Breast Surgery, Qilu Hospital of Shandong University, a small number of cases may influence the representativeness of two groups. Further researches including multi-center studies are still necessary for better understanding the relationship between multiple clinical factors and breast cancer with nipple discharge. Nonetheless, not all patients with nipple discharge had all five tumor markers analyzed at one time, due primarily to the identification of enrolled markers at various time points during the study. We didn’t have a follow-up of the patient’s prognosis and survival rates. So whether CEA and CA153 could be used as prognostic factors of breast cancer is to be discussed. Further investigation on the correlation between screening, disease progress, follow-up monitoring and treatment response is required.
In conclusion, based on data in the present study, tumor markers including CEA and CA153 may be the efficient, cost-effective diagnostic method in breast cancer. It is unreliable to use CA199, CA724 and AFP to predict breast cancer. In general, it is suggested that the combination of CEA, CA153, age and palpable masses in physical examination is of clinical value for distinguishing between breast cancer and other benign diseases. As a potential routine preoperative examination, detection of CEA and CA153 in nipple discharge may improve clinical decisions and play important clinical roles in the early diagnosis, treatment of breast cancer.
Acknowledgements
We thank members of Department of Breast Surgery, Qilu Hospital of Shandong University for their advice on the research: Drs. Qifeng Yang, Xiao Wang, Qing Wu, Huantao Liu.
Disclosure of conflict of interest
None.
References
- 1.Vargas HI, Romero L, Chlebowski RT. Management of bloody nipple discharge. Curr Treat Options Oncol. 2002;3:157–161. doi: 10.1007/s11864-002-0061-9. [DOI] [PubMed] [Google Scholar]
- 2.Louie LD, Crowe JP, Dawson AE, Lee KB, Baynes DL, Dowdy T, Kim JA. Identification of breast cancer in patients with pathologic nipple discharge: does ductoscopy predict malignancy? Am J Surg. 2006;192:530–533. doi: 10.1016/j.amjsurg.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Montroni I, Santini D, Zucchini G, Fiacchi M, Zanotti S, Ugolini G, Manaresi A, Taffurelli M. Nipple discharge: is its significance as a risk factor for breast cancer fully understood? Observational study including 915 consecutive patients who underwent selective duct excision. Breast Cancer Res Treat. 2010;123:895–900. doi: 10.1007/s10549-010-0815-1. [DOI] [PubMed] [Google Scholar]
- 4.Lamerz R. CEA determination in the follow-up of extracolorectal neoplasms. Int J Biol Markers. 1992;7:171–178. doi: 10.1177/172460089200700309. [DOI] [PubMed] [Google Scholar]
- 5.Woo SJ, Kim CH, Park MY, Kim HS, Sohn HJ, Park JS, Kim HJ, Oh ST, Kim TG. Co-administration of carcinoembryonic antigen and HIV TAT fusion protein with CpG-oligodeoxynucleotide induces potent antitumor immunity. Cancer Sci. 2008;99:1034–1039. doi: 10.1111/j.1349-7006.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Cao H, Jiao Z, Pakala SB, Sirigiri DN, Li W, Kumar R, Mishra L. Carcinoembryonic antigen interacts with TGF-{beta} receptor and inhibits TGF-{beta} signaling in colorectal cancers. Cancer Res. 2010;70:8159–8168. doi: 10.1158/0008-5472.CAN-10-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Y, Wang L, Zhang P, Wei H, Gao R, Liu X, Yu Y, Wang L. Detection of circulating anti-mucin 1 (MUC1) antibodies in breast tumor patients by indirect enzyme-linked immunosorbent assay using a recombinant MUC1 protein containing six tandem repeats and expressed in Escherichia coli. Clin Vaccine Immunol. 2010;17:1903–1908. doi: 10.1128/CVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blixt O, Bueti D, Burford B, Allen D, Julien S, Hollingsworth M, Gammerman A, Fentiman I, Taylor-Papadimitriou J, Burchell JM. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13:R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BK, Lee JW, Park PJ, Shin YS, Lee WY, Lee KA, Ye S, Hyun H, Kang KN, Yeo D, Kim Y, Ohn SY, Noh DY, Kim CW. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009;11:R22. doi: 10.1186/bcr2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarcione EJ, Biddle W. Elevated serum alpha fetoprotein levels in postmenopausal women with primary breast carcinoma. Dis Markers. 1987;5:75–79. [PubMed] [Google Scholar]
- 11.Benn PA. Advances in prenatal screening for Down syndrome: I. general principles and second trimester testing. Clin Chim Acta. 2002;323:1–16. doi: 10.1016/s0009-8981(02)00186-9. [DOI] [PubMed] [Google Scholar]
- 12.Kagan KO, Wright D, Spencer K, Molina FS, Nicolaides KH. First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: impact of maternal and pregnancy characteristics. Ultrasound Obstet Gynecol. 2008;31:493–502. doi: 10.1002/uog.5332. [DOI] [PubMed] [Google Scholar]
- 13.Chen XZ, Zhang WK, Yang K, Wang LL, Liu J, Wang L, Hu JK, Zhang B, Chen ZX, Chen JP, Zhou ZG, Mo XM. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39:9031–9039. doi: 10.1007/s11033-012-1774-x. [DOI] [PubMed] [Google Scholar]
- 14.Bian J, Li B, Kou XJ, Liu TZ, Ming L. Clinical significance of combined detection of serum tumor markers in diagnosis of patients with ovarian cancer. Asian Pac J Cancer Prev. 2013;14:6241–6243. doi: 10.7314/apjcp.2013.14.11.6241. [DOI] [PubMed] [Google Scholar]
- 15.Bian J, Li B, Kou XJ, Wang XN, Sun XX, Ming L. Clinical applicability of multi-tumor marker protein chips for diagnosing ovarian cancer. Asian Pac J Cancer Prev. 2014;15:8409–8411. doi: 10.7314/apjcp.2014.15.19.8409. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Niu L, Chiu D, He L, Xu K. Changes in the expression of serum markers CA242, CA199, CA125, CEA, TNF-alpha and TSGF after cryosurgery in pancreatic cancer patients. Biotechnol Lett. 2012;34:1235–1241. doi: 10.1007/s10529-012-0908-5. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng CW, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H, Crane CH, Wolff RA, Varadhachary GR, Pisters PW, Aloia TA, Vauthey JN, Fleming JB, Katz MH. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430–438. doi: 10.1111/hpb.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pengjun Z, Xinyu W, Feng G, Xinxin D, Yulan L, Juan L, Xingwang J, Zhennan D, Yaping T. Multiplexed cytokine profiling of serum for detection of colorectal cancer. Future Oncol. 2013;9:1017–1027. doi: 10.2217/fon.13.71. [DOI] [PubMed] [Google Scholar]
- 19.Xiong GP, Zhang JX, Gu SP, Wu YB, Liu JF. Overexpression of ECM1 contributes to migration and invasion in cholangiocarcinoma cell. Neoplasma. 2012;59:409–415. doi: 10.4149/neo_2012_053. [DOI] [PubMed] [Google Scholar]
- 20.Park IJ, Choi GS, Jun SH. Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res. 2009;29:4303–4308. [PubMed] [Google Scholar]
- 21.Gaspar MJ, Arribas I, Coca MC, Diez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001;22:318–322. doi: 10.1159/000050633. [DOI] [PubMed] [Google Scholar]
- 22.Guadagni F, Roselli M, Cosimelli M, Ferroni P, Spila A, Cavaliere F, Casaldi V, Wappner G, Abbolito MR, Greiner JW, et al. CA 72-4 serum marker--a new tool in the management of carcinoma patients. Cancer Invest. 1995;13:227–238. doi: 10.3109/07357909509011692. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 24.Bennett JA, Semeniuk DJ, Jacobson HI, Murgita RA. Similarity between natural and recombinant human alpha-fetoprotein as inhibitors of estrogen-dependent breast cancer growth. Breast Cancer Res Treat. 1997;45:169–179. doi: 10.1023/a:1005841032371. [DOI] [PubMed] [Google Scholar]
- 25.Richardson BE, Hulka BS, Peck JL, Hughes CL, van den Berg BJ, Christianson RE, Calvin JA. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am J Epidemiol. 1998;148:719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 26.Melbye M, Wohlfahrt J, Lei U, Norgaard-Pedersen B, Mouridsen HT, Lambe M, Michels KB. alpha-fetoprotein levels in maternal serum during pregnancy and maternal breast cancer incidence. J Natl Cancer Inst. 2000;92:1001–1005. doi: 10.1093/jnci/92.12.1001. [DOI] [PubMed] [Google Scholar]


