Abstract
Background
Changes in the mode of aerobic energy production are observed in many solid tumors, though the kinds of changes differ among tumor types. We investigated mitochondrial energy metabolism in meningiomas and peripheral nerve sheath tumors, taking into consideration the histologic heterogeneity of these tumors.
Methods
Oxidative phosphorylation (OXPHOS) complexes and porin (a marker for mitochondrial mass) were analyzed by immunohistochemical staining of meningiomas (n = 76) and peripheral nerve sheath tumors (schwannomas: n = 10; neurofibromas: n = 4). The enzymatic activities of OXPHOS complexes and citrate synthase were determined by spectrophotometric measurement. Western blot analysis of OXPHOS complexes, porin, and mitochondrial transcription factor A was performed. Furthermore, mitochondrial DNA copy number was determined.
Results
The tumors differed with regard to mitochondrial energy metabolism. Low levels of a subset of OXPHOS complexes were frequently observed in World Health Organization grade I meningiomas (percent of cases with a reduction; complex I: 63%; complex II: 67%; complex IV: 56%) and schwannomas (complex III: 40%, complex IV: 100%), whereas in neurofibromas a general reduction of all complexes was observed. In contrast, expression of complexes III and V was similar to that in normal brain tissue in the majority of tumors. Mitochondrial mass was comparable or higher in all tumors compared with normal brain tissue, whereas mitochondrial DNA copy number was reduced.
Conclusions
The reduction of OXPHOS complexes in meningiomas and peripheral nerve sheath tumors has potential therapeutic implications, since respiratory chain–deficient tumor cells might be selectively starved by inhibitors of glycolysis or by ketogenic diet.
Keywords: meningioma, neurofibroma, oxidative phosphorylation, respiratory chain deficiency, schwannoma
Tumors of the meninges account for approximately a third of all primary intracranial tumors. Meningiomas are probably derived from arachnoidal cap cells residing in the arachnoid layer.1 Generally, meningiomas are benign, slow-growing tumors with good prognosis. Considerably more aggressive, however, are rare variants (eg, clear cell) as well as brain-invasive (World Health Organization [WHO] grade II), atypical (grade II), and anaplastic (grade III) meningiomas.2
The etiology of meningiomas is unknown. However, it has been shown that neurofibromatosis type 2 (NF2), an autosomal dominant disorder, is associated with meningiomas and benign peripheral nerve sheath tumors (PNSTs).3
Histological subtypes of grade I meningiomas are distinguished and include, among others, meningothelial, fibrous, transitional, psammomatous, and secretory meningiomas. Meningothelial meningiomas are histologically composed of characteristic uniform tumor cells that form lobules surrounded by thin collagenous septae. Fibrous meningiomas are mainly composed of spindle-shaped cells that resemble fibroblasts and form intersecting fascicles embedded in a collagen-rich and reticulin-rich matrix. Transitional (mixed) meningiomas combine features of both subtypes. Transitional and meningothelial meningiomas have a tendency to both hyalinize and calcify, forming characteristic concentric calcifications known as psammoma bodies. Tumors composed mostly of the latter are referred to as psammomatous meningiomas.4
PNSTs arise from peripheral nerves and show nerve sheath differentiation. Several distinct benign tumor types have been recognized, including schwannoma, neurofibroma, and perineurioma.
The aerobic use of glucose as an energy source through glycolysis is a common feature of most solid tumors, leading to reduced dependence on oxidative phosphorylation (OXPHOS), a phenomenon known as the Warburg effect.5 The OXPHOS system is composed of NADH:ubiquinone oxidoreductase (complex I), succinate dehydrogenase (SDH; complex II), coenzyme Q cytochrome c oxidase (complex III), cytochrome c oxidase (COX; complex IV), ATP synthase (complex V), and 2 electron carriers, namely cytochrome c and coenzyme Q. Downregulation of OXPHOS in tumor cells is achieved by, among others, the following mechanisms: (i) lack of vascularization in rapidly growing tumors leading to profound hypoxia, which causes a compensatory upregulation of glycolysis6; (ii) genetic inactivation of regulators of OXPHOS, such as the von Hippel Lindau (VHL) and p53 genes, as well as activation of oncogenes resulting in downregulation of OXPHOS6–8; and (iii) direct genetic inactivation of components of OXPHOS. Loss of complex I of OXPHOS was shown to be associated with oxyphilic tumors.9–11 In addition, pheochromocytomas and paragangliomas frequently exhibit mutations in SDH genes, indicating that SDH subunits act as tumor suppressors in neuroendocrine tissues.12
The aim of the present study was to evaluate alterations of aerobic mitochondrial energy metabolism in meningiomas and PNSTs.
Materials and Methods
Samples
Tumors of the meninges (n = 76) and PNSTs (n = 14) (schwannomas [n = 10]; neurofibromas [n = 4]) were examined. Histomorphologically normal brain areas adjacent to the tumors as determined by a neuropathologist from 6 meningiomas and 38 neuroepithelial brain tumor patients were used as controls (Table 1 and Supplementary Table 1).13 The tumors were classified according to WHO criteria.14 For the immunohistochemical studies, formalin-fixed, paraffin-embedded tissues were used. For spectrophotometric analysis of OXPHOS enzyme and citrate synthase activity, 38 frozen tissues were available. The histologies of the frozen tissues are given in Supplementary Table 1. In addition, control brain tissue from healthy autopsies was taken for comparison of enzymatic measurements and western blot analysis. The controls were aged between 50 and 87 years (3 males, 2 females). The study was performed according to the Austrian Gene Technology Act. Experiments were performed in accordance with the Helsinki Declaration of 1975 (revised 2000) and the guidelines of the Salzburg State Ethics Research Committee, being no clinical drug trial or epidemiological investigation. Patients signed consent for scientific evaluation of the tumor tissue. Furthermore, the study did not extend to examination of individual case records. Patient anonymity was ensured at all times.
Table 1.
Mean values of the staining intensities of porin and the OXPHOS complexes in meningiomas and normal brain tissues
| Tissue (grade) | n | Porin | Complex I | Complex II | Complex III | Complex IV | Complex V | MtDNA Copy Number |
|
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | n | Mean ± SEM | ||
| Brain control | 44 | 2.1 ± 0.1 | 2.2 ± 0.1 | 1.5 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 | 17 | 3486 ± 455 |
| Fibroblastic meningioma (I) | 17 | 1.8 ± 0.1 | 1.2 ± 0.2** | 0.9 ± 0.2* | 2.3 ± 0.1 | 1.2 ± 0.3** | 2.2 ± 0.2 | 17 | 797 ± 337** |
| Transitional meningioma (I) | 16 | 2.0 ± 0.1 | 1.0 ± 0.1** | 1.3 ± 0.2 | 2.5 ± 0.1 | 1.4 ± 0.2** | 2.6 ± 0.1* | 16 | 1244 ± 491** |
| Meningothelial meningioma (I) | 17 | 1.9 ± 0.1 | 1.4 ± 0.2* | 0.9 ± 0.1** | 2.3 ± 0.1 | 1.6 ± 0.2** | 2.4 ± 0.1 | 17 | 1241 ± 328** |
| Psammomatous meningioma (I) | 4 | 1.9 ± 0.1 | 1.3 ± 0.6 | 1.1 ± 0.5 | 1.6 ± 0.6* | 1.1 ± 0.6* | 1.5 ± 0.5 | 3 | 1365 ± 568* |
| Secretory meningioma (I) | 3 | 2.2 ± 0.3 | 0.5* | 2.2 ± 0.6 | 2.7 ± 0.3 | 0.8 ± 0.2* | 2.3 ± 0.2 | 3 | 1907 ± 723 |
| Atypical meningioma (II) | 11 | 1.9 ± 0.1 | 1.8 ± 0.2 | 1.2 ± 0.2 | 2.4 ± 0.1 | 2.3 ± 0.2 | 2.7 ± 0.1* | 11 | 928 ± 146** |
| Anaplastic meningioma (III) | 8 | 2.0 ± 0.2 | 1.7 ± 0.4 | 2.1 ± 0.3* | 2.5 ± 0.3 | 1.6 ± 0.4 | 2.6 ± 0.2* | 8 | 1060 ± 444* |
| Schwannoma (I) | 10 | 2.8 ± 0.1* | 1.8 ± 0.6 | 1.6 ± 0.3 | 1.5 ± 0.2** | 0.6 ± 0.1** | 1.8 ± 0.2 | 8 | 863 ± 310** |
| Neurofibroma (I) | 4 | 2.4 ± 0.8 | 0.3 ± 0.1** | 0.9 ± 0.4* | 1.6 ± 0.3* | 0.6 ± 0.1** | 0.9 ± 0.2** | 4 | 437 ± 109* |
*P < .05, **P < .001.
Immunohistochemical Staining and Analysis
The following antibodies were used: complex I subunit NADH dehydrogenase:ubiquinone Fe-S protein 4 (NDUFS4; mouse monoclonal, 1:1000; Abcam), complex II subunit 70 kDa Fp (mouse monoclonal, 1:2000; MitoSciences), complex III subunit core 2 (mouse monoclonal, 1:1500; MitoSciences), complex IV subunit I (mouse monoclonal, 1:1000; MitoSciences), complex V subunit alpha (mouse monoclonal, 1:2000; MitoSciences), and porin 31HL (mouse monoclonal, 1:3000; MitoSciences). All antibodies were diluted in Dako antibody diluent with background-reducing components. Immunohistochemical staining was performed as described previously.11 The staining intensities of the tumors were determined by 2 independent examiners blinded to the diagnosis. Staining intensities were rated using a 0–3 scoring system, with 0 representing no staining, 1 mild, 2 moderate, and 3 strong staining.
Spectrophotometric Measurement of OXPHOS Enzyme and Citrate Synthase Activity
Spectrophotometric measurement of OXPHOS enzyme and citrate synthase activity was performed as previously described.13,15 Tumor tissues (20–100 mg) were homogenized in extraction buffer (20 mM Tris-HCl, pH 7.6, 250 mM sucrose, 40 mM KCl, 2 mM EGTA). The postnuclear supernatant (600 g homogenate) containing the mitochondrial fraction was used for measurement of enzyme activities and western blot analysis.
Western Blot Analysis
Ten micrograms of protein of 600 g homogenate was separated on acrylamide/bisacrylamide gels and transferred to nitrocellulose membranes. Immunological detection of proteins was carried out as described previously.13 The following primary antibodies were used: monoclonal mouse anti-NDUFS4 (Abcam); monoclonal mouse anti-TFAM (mitochondrial transcription factor A; Abcam); monoclonal mouse anti–SDH subunit A (SDHA) 70 kD antibody (MitoSciences), monoclonal mouse anti–core 2 antibody (MitoSciences), monoclonal mouse antiporin antibody (MitoSciences), and polyclonal rabbit anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Trevigen).
Determination of Mitochondrial DNA Alterations
Mitochondrial (mt)DNA copy number and the amount of the common mitochondrial deletion were determined by quantitative real-time (qRT) PCR of 2 mtDNA regions as previously described (Supplementary Table 1).16 In addition, the genes of the mitochondrially encoded subunits of complexes I and IV and transfer RNA genes were sequenced in samples that showed staining intensities of OXPHOS complexes (I and IV) ≤1 (Supplementary Table 1). Details about the primers used for PCR amplification and sequence analysis are given in Supplementary Table 2. Sequences were compared with the complete Homo sapiens mitochondrial genome (GenBank sequence J01415).
Statistical Analysis
Data were analyzed with PASW statistics 18. Student's t-test and the nonparametric Mann-Whitney U-test were applied for comparison of tumors and normal tissues as well as among tumor grades. To simplify the analysis, we grouped the intensities as 0, 0.5, and 1 (low), 1.5 and 2 (moderate), and 2.5 and 3 (high). For correlations of the OXPHOS enzyme expression, mtDNA copy number, and tumor grades, the nonparametric Spearman correlation was used.
Results
Immunohistochemical analysis of porin and the 5 OXPHOS complexes were performed in order to evaluate the status of aerobic mitochondrial energy metabolism in meningiomas and PNSTs. Examples for staining intensities 1–3 (0 = no expression, 1 = weak, 2 = moderate, 3 = strong) of porin and the 5 OXPHOS complexes are shown in Figs 1 and 2. To investigate whether the changes in OXPHOS enzyme expression have functional consequences, the enzymatic activities of citrate synthase and the OXPHOS complexes were measured in a subset of the samples (Fig. 3A–F).
Fig. 1.
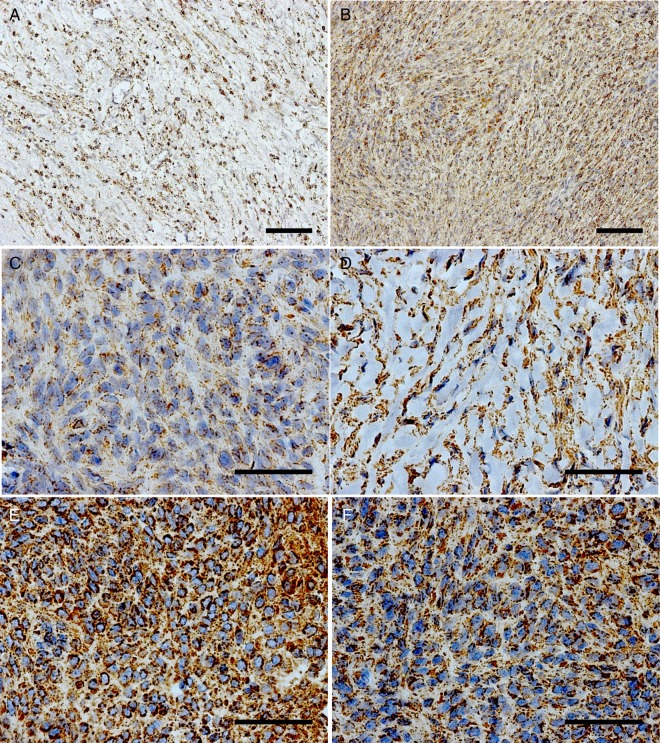
Porin staining of meningiomas with a high and low content of connective tissue and/or cytoplasmic matrix components. (A and B) Transitional meningioma (TM1). (A) Region with punctuate cytoplasmic reactivities (staining intensity = 2). (B) Region with a sparse connective tissue content (staining intensity = 2); 20× magnification. (C) Area with a low connective tissue content from a fibroblastic meningioma (FM4) and a weak expression of porin (staining intensity = 1). (D) High connective tissue content but strong porin expression in an area of a meningothelial meningioma (MM4) (staining intensity = 2.5). (E) Low connective tissue content and high porin expression in an area of an atypical meningioma (AM1) (staining intensity = 3). (F) Low connective tissue content and high porin expression (staining intensity = 2) in an area of an anaplastic meningioma (ANM2); (C–F) 63× magnification. (A and B) Scale bar = 100 µm; (C–F) scale bar = 50 µm.
Fig. 2.
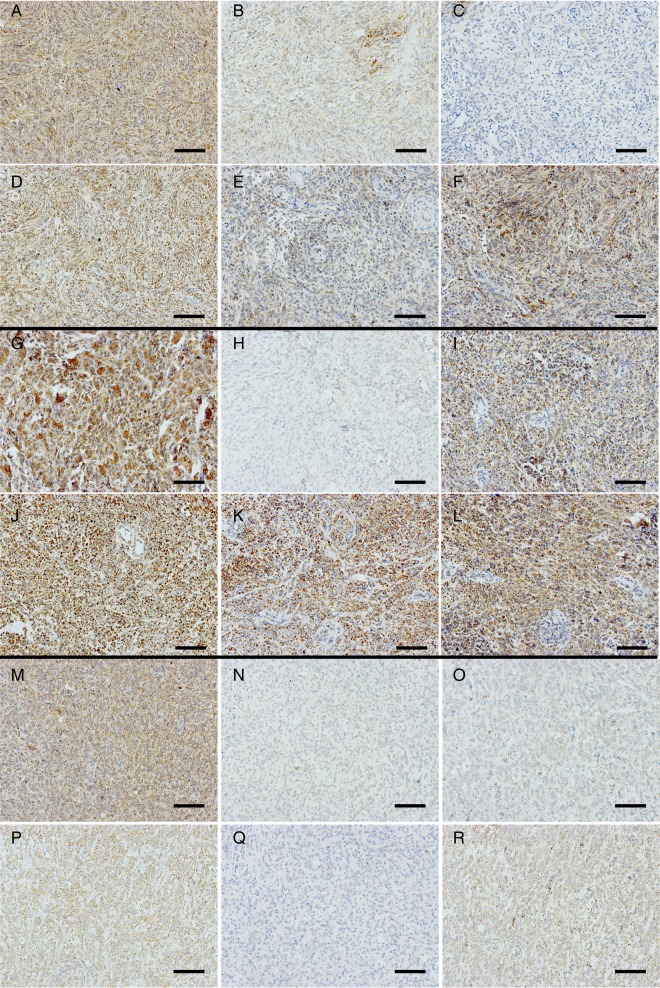
Expression of OXPHOS complexes in WHO grade I (meningothelial), II (atypical), and III (anaplastic) meningiomas. (A, G, and M) porin; (B, H, and N) complex I; (C, I, and O) complex II; (D, J, and P) complex III; (E, K, and Q) complex IV; (F, L, and R) complex V. (A–F) Meningothelial meningioma (MM1) with a complex II deficiency and a reduction of complexes I and IV (Supplementary Table 1). (G–L) Atypical meningioma (AM4) with an isolated complex I deficiency (Supplementary Table 1). (M–R) Combined low complexes I, II, and IV in an anaplastic meningioma (ANM1). (H) Staining intensity = 0; 20× magnification. Scale bar = 100 µm.
Fig. 3.
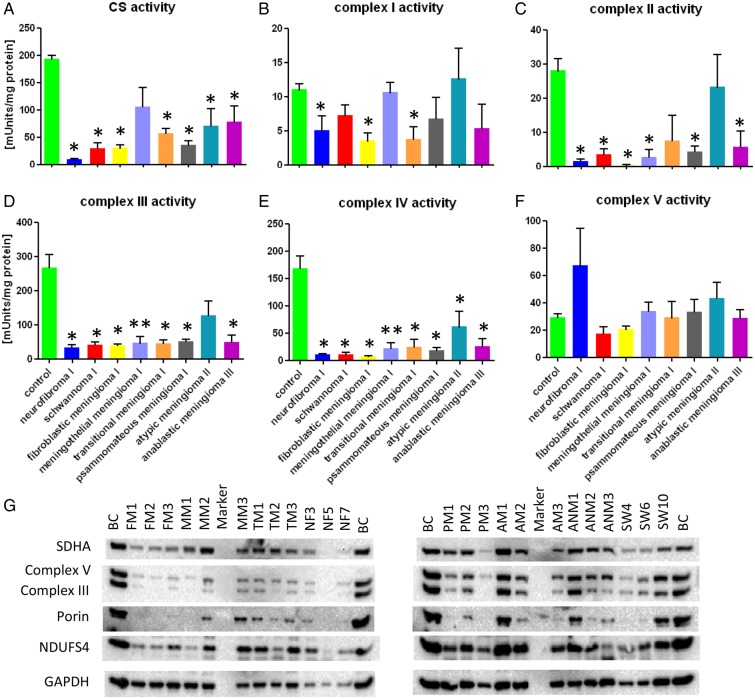
Enzymatic activities of the OXPHOS complexes and the citrate synthase in meningiomas, PNSTs, and control brain tissue. (A) Citrate synthase (CS) activity; (B) complex I activity; (C) complex II activity; (D) complex III activity; (E) complex IV activity; (F) complex V activity. Absolute enzyme activities are given in mUnits/mg protein. The activities were compared with normal brain tissue. Values are given as mean ± SEM. *P < .05; **P < .01. (G) Immunoblot analysis of meningiomas and PNSTs (for details on tumors, see Supplementary Table 1) and control brain tissue. BC: control brain; n = 5; NF: n = 4; SW: n = 4; FM: n = 4; MM: n = 5; TM: n = 4; PM: n = 4; AM: n = 5; ANM: n = 3.
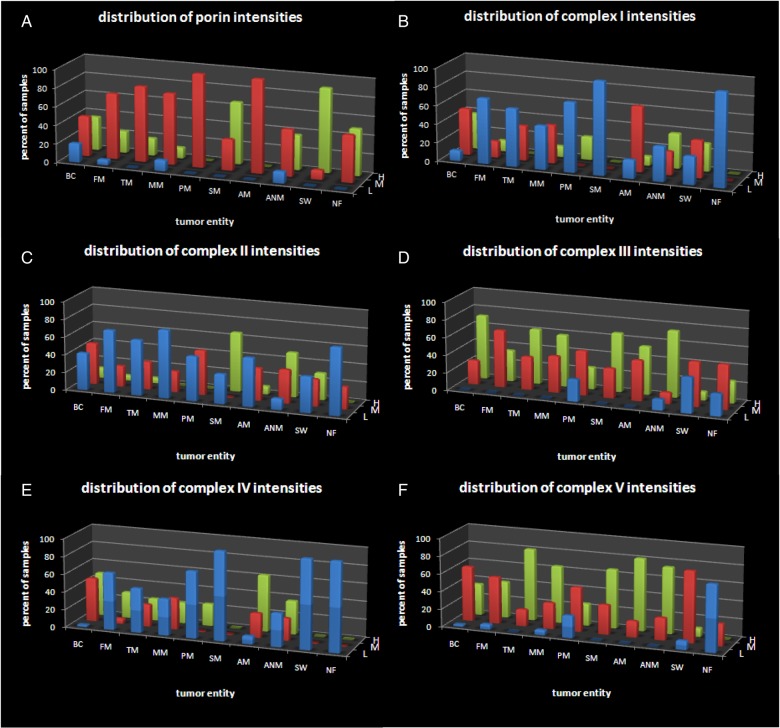
We determined the frequencies of the staining intensities in meningiomas and PNSTs. As shown in Fig. 3, the distributions for porin, complex III, and complex V in meningiomas are generally similar to those of the control brain tissue. For complexes I, II, and IV, the distributions are skewed toward lower staining intensities in the tumors (Fig. 4).
Fig. 4.
Distribution of the staining intensities in meningiomas, PNSTs, and normal brain tissue. (A) Porin, (B) complex I, (C) complex II, (D) complex III, (E) complex IV, (F) complex V. BC, control brain; FM, fibroblastic meningioma; TM, transitional meningioma; MM, meningothelial meningioma; PM, psammomatous meningioma; SM, secretory meningioma; AM, atypical meningioma; ANM, anaplastic meningioma; SW, schwannoma; NF, neurofibroma. L, low expressors (blue) (staining intensities = 0–1); M, moderate expressors (red) (staining intensities = 1.5–2); H, high expressors (green) (staining intensities = 2.5–3).
Meningothelial Meningioma (WHO Grade I)
Significantly lower levels of complexes I (P < .05), II (P < .01), and IV (P < .01) were observed by immunohistochemistry, with no difference in mitochondrial mass compared with control tissue (Figs 1, 2, and 4 and Table 1). Total citrate synthase activity was higher in meningothelial meningiomas compared with fibroblastic and transitional meningiomas (Fig. 3A), indicating that the content of the connective tissue/cytoplasmic matrix component in meningothelial meningiomas is not as high as in other low-grade meningiomas. Western blot analysis of porin revealed relatively high expression in 2 of the 3 meningothelial meningiomas examined compared with other low-grade tumors. Complex I activity did not differ from that of control brain tissue (Fig. 3A and B).
Fibroblastic Meningioma (WHO Grade I)
Significantly reduced expression of complexes I (P < .01), II (P < .05), and IV (P < .01) was detected in fibroblastic meningiomas compared with control brain tissue by immunohistochemical analysis (Table 1; Fig. 4). No alterations of porin or complexes III and V were found. A general reduction in the total activities of citrate synthase and all OXPHOS complexes (with the exception of complex V) was found in fibroblastic meningiomas (Fig. 3). Western blot analysis revealed a general reduction in the amounts of porin and OXPHOS complexes (Fig. 3G). The somewhat contradictory results between the immunohistochemical data and the enzymatic measurements might be due to the high connective tissue content of fibroblastic meningiomas, which constitutes a substantial amount of the protein in the lysates.
Transitional Meningioma (WHO Grade I)
Similar to fibroblastic meningiomas (Fig. 1C and D), the connective tissue content of transitional meningiomas is highly variable (Fig. 1A and B). Compared with normal brain tissue, no alterations of mitochondrial mass were observed in transitional meningiomas, as indicated by porin staining (Table 1). In contrast, a significant reduction of complexes I (P < .01) and IV (P < .01) was found in these tumors, whereas complex V was increased. The enzymatic activities of citrate synthase and OXPHOS complexes I, III, and IV were reduced in transitional meningiomas (Fig. 3). Complex II showed a trend to lower levels compared with control tissue. Complex V activity was equal in fibroblastic meningiomas and normal brain tissue.
Psammomatous Meningioma (WHO Grade I)
Significantly lower expression of complexes III (P < .05) and IV (P < .05) was found in psammomatous meningiomas (Table 1). However, a trend to lower levels was also observed for complexes I and V. The exclusively nucleus-encoded complex II was not affected. This is underlined by immunoblot analysis (Fig. 3G), where 2 of 3 psammomatous meningiomas showed high complex II expression. The low activity of citrate synthase and the OXPHOS complexes observed in these tumors might be due to the presence of psammoma bodies.
Secretory Meningioma (WHO Grade I)
A significant reduction of complexes I and IV was obvious in secretory meningiomas (Table 1; Fig. 4). No frozen tissues were available for enzymatic and western blot analysis.
Atypical Meningioma (WHO Grade II)
The expression levels of porin and OXPHOS complexes in WHO grade II meningiomas (atypical meningiomas) were similar to those in controls (Table 1; Fig. 2). Enzymatic measurements revealed a significant reduction in citrate synthase activity (Fig. 3A). Activities of complexes I, III, and V were normal, whereas those of complexes II and IV were reduced (Fig. 3A). Consistent with these findings, complex I expression determined by western blot analysis was higher in atypical meningiomas compared with that in other tumor entities and normal control tissue (Fig. 3G).
Anaplastic Meningioma (WHO Grade III)
Levels of porin and OXPHOS complexes I, III, and IV in anaplastic meningiomas (WHO grade III) were comparable to those in normal brain tissue (Figs 2 and 4). Complex II expression was significantly upregulated in anaplastic meningiomas in comparison with both WHO grades I (P = .002) and II (P = .035) meningiomas as well as normal brain tissue. Complex V was significantly upregulated compared with control tissue (Table 1). Enzymatic measurements revealed a general downregulation of citrate synthase and OXPHOS complexes I–IV (Fig. 3A–E). Complex V activity (Fig. 3F) was within the normal range.
Correlation of the OXPHOS Complexes to Meningioma Tumor Grade
A significant positive correlation was found among expression levels of complex I (P = .0087, R = 0.3008), complex II (P = .0124, R = 0.2855), complex IV (P = .0295, R = 0.2498), and complex V (P = .0154, R = 0.2769) by immunohistochemical analysis and meningioma grade. In addition, total (P = .0113, R = 0.4980) as well as the citrate synthase normalized activity of complex II (P = .0183, R = 0.4930) positively correlated with tumor grade.
Schwannoma (WHO Grade I)
The mitochondrial mass (porin staining) was increased in schwannomas. All OXPHOS complexes were reduced, with exception of the exclusively nuclear-encoded complex II (Table 1). MtDNA copy number was significantly reduced compared with control brain tissue (Table 1). Enzymatic measurement revealed downregulation of citrate synthase activity and the OXPHOS complexes II, III, and IV. Complexes I and V showed only a trend to lower activities (Fig. 3).
Neurofibromas (WHO Grade I)
A high mitochondrial mass was found in neurofibromas (Table 1). All OXPHOS complexes and mtDNA copy number were significantly reduced compared with brain controls (Table 1). Citrate synthase and the activity of complexes I–IV were significantly reduced. Complex V activity was increased (Fig. 3).
Genetics
MtDNA copy number determined by qRT-PCR was significantly diminished (P < .01) in all meningioma entities and PNSTs (Table 1). Since mtDNA deletions can cause combined or general reductions of OXPHOS complexes, all meningiomas and PNSTs were analyzed by qRT-PCR. There were no significant differences in the cycle threshold values of the 2 mitochondrial PCR products of either the COX1 or COX3/NADH dehydrogenase subunit 3 (ND3) genes, excluding a highly prevalent (<50%) common deletion in the analyzed tumors.
Sequence analysis of tumors that showed staining intensities of OXPHOS complexes ≤1 revealed a novel nonsynonymous mtDNA mutation in one transitional meningioma (case TM6; Supplementary Table 1). Unfortunately, no matched control tissue was available. The identified mutation (m.13843G > A) affects an amino acid residue with low conservation in the ND5 protein.
Sequencing of nuclear genes encoding SDH subunits (B, C, and D) in meningothelial meningiomas exhibiting isolated reductions in complex II revealed no pathogenic mutations.
Discussion
Normal mitochondrial mass was observed in meningiomas and PNSTs regardless of the WHO grade, determined by immunohistochemical analysis. The normal mitochondrial mass is in agreement with ultrastructural studies reporting abundant mitochondria in meningiomas.17 The mtDNA reduction found in meningiomas and PNSTs could contribute to the reduction of the OXPHOS complexes. A high mitochondrial mass but low mtDNA copy number is typical for patients suffering from mtDNA depletion syndromes. Since the number of mitochondrially encoded subunits differs (eg, complex II exclusively nuclear encoded) among the OXPHOS complexes, an mtDNA depletion can differentially affect the OXPHOS enzymes. Low levels of citrate synthase activity, a marker enzyme for mitochondrial mass, were found in meningiomas. These findings are consistent with data reported by Herting et al.18 A low expression of the citrate synthase was also described recently by Sharma et al (2015).19 PNSTs also exhibited a reduced citrate synthase activity. Generally, a decrease of citrate synthase activity indicates a reduction of mitochondrial mass usually paralleled by a reduction in the amounts of all OXPHOS complexes.20 However, the low citrate synthase activity found in meningiomas and PNSTs is mainly due to the high amount of connective tissue and/or cytoplasmic matrix components. For example, fibroblastic meningiomas synthesize large amounts of collagen and reticulin that significantly contribute to the protein concentration determined in tissue extracts when calculating absolute enzyme activities; large amounts of connective tissue and/or cytoplasmic matrix components lead to an underestimation of absolute enzymatic activities. As the mitochondrial mass determined by porin staining in meningioma and PNST cells is similar to that of control tissue, the diminished citrate synthase activity cannot be explained by changes of the mitochondrial mass. Hence, immunohistochemistry is more suitable to elucidate the level of certain proteins in tumor tissues to be able to directly compare the expression of tumor cells and nontumor cells, whereas techniques like western blot analysis and enzymatic analysis using frozen tissue specimens can determine only the overall content/activity of a protein. Especially in inhomogeneous tumors, functional analysis does not allow assertion regarding alterations in tumor cells only.
A reduction of complex I in meningiomas and neurofibromas compared with normal brain tissue was detected by immunohistochemical analysis. Since neurons are used as the reference cell type, this finding should be considered critically, because it is supposed that meningiomas originate from nonneuronal, arachnoidal cap cells. In the study by Herting and colleagues, upregulation of complex I activity was detected in meningiomas,18 whereas we found significantly lower absolute activity of complex I in fibroblastic and transitional meningiomas, which is in agreement with the study by Sharma et al (2015), who reported a reduced expression of numerous complex I subunits.19 However, complex I activity normalized to citrate synthase activity was higher in all analyzed meningioma entities compared with brain tissue.
The total complex III and IV activity was lower in most meningioma entities compared with control brain tissue. Again, the reduction of these complexes is likely due to the high amount of connective tissue and/or cytoplasmic matrix components. Immunohistochemical analysis revealed a downregulation of complex IV in meningiomas (with the exception of atypical meningiomas), which is consistent with our enzymatic analysis. In agreement, complex II was reduced in low-grade meningiomas. Interestingly, we found a significant positive correlation between complex II expression as well as complex II activity and meningioma tumor grade. This is in agreement with recently reported reduced levels of the SDHA subunit in low-grade meningiomas and normal levels in atypical meningiomas.19 Expression of complexes I, IV, and V in tumor cells also significantly correlated with tumor grade.
The respiratory chain complexes are organized into supercomplexes. Complexes I, III, and IV are assembled into supramolecular structures.21 Since complex IV is required for assembly and stability of complex I,22 loss of complex IV can lead to a secondary reduction of all other OXPHOS complexes. Recently, several studies identified factors that cause combined OXPHOS deficiencies. Interestingly, depending on the affected protein, different OXPHOS defects result.23–25 We propose that alterations of an unknown etiological factor lead to the exclusive reduction of complexes I, II, and IV of the respiratory chain, as observed in meningiomas.
It is becoming more and more evident that several signaling pathways affecting mitochondrial function play a role in tumor development and progression. In meningiomas and PNSTs, many frequently affected proteins belong to the same energy metabolism network. Dysregulation of any of these factors could contribute to the Warburg effect and thereby contribute to tumorigenesis in meningiomas/PNSTs.
The frequencies of chromosome losses in WHO grade II meningiomas are: 22q (85%), 14q (60%), 1p (55%), 6q (25%), and 18q (20%).26 Guan et al27 detected a candidate common loss-of-heterozygosity region on 1p36.11. Monosomy 1p is correlated with enhanced in vivo glucose metabolism in meningiomas.28 The SDHB gene lies within this region. Loss of complex II leads to the accumulation of succinate, which in turn causes stabilization of hypoxia-inducible factor 1α (HIF-1α).29 HIF-1α is a master regulator of energy metabolism and known to be responsible for the glycolytic switch in tumor cells, thus providing a possible link between monosomy 1p and increased glycolysis in meningioma cells. Accordingly, HIF-1α is elevated in transitional meningiomas.30 Several transcriptional targets of HIF-1α are also elevated in meningiomas—for example, the hypoxia marker carbonic anhydrase 9 in anaplastic meningiomas.31 The VHL protein, which has ubiquitin E3 ligase activity, regulates the degradation of HIF-1α. VHL deletions were detected in 12 of 15 meningiomas of different WHO grades.32 A decrease of OXPHOS complexes was observed in tumors with VHL mutations.33 Recently it was shown that transfection of VHL-deficient renal cell carcinoma cell lines with VHL causes an upregulation of the expression of OXPHOS complexes.34 Meningiomas are well-vascularized tumors, which might also be explained by loss of VHL. Targeted inactivation of VHL leads to the development of highly vascularized tumors.35 The fact that blood supply, and therefore oxygen delivery to meningiomas, presumably is high suggests that the Warburg effect and HIF-1α stabilization in meningiomas are not induced by hypoxia but possibly via a VHL-dependent mechanism.
Recently it was shown that p53, which is frequently mutated in meningiomas, is a secondary master regulator of energy metabolism besides HIF-1α.36 Loss of p53 can cause mtDNA depletion and can regulate expression of COX 2 (synthesis of cytochrome oxidase 2).37 Telomerase activity has been detected in most cancers, suggesting a role for telomerase in supporting the proliferative capacity of cells progressing toward malignancy. It is hypothesized that reactivation of telomerase is required to maintain tumor growth. No telomerase activity is present in benign meningiomas.38 Mitochondrial telomerase reverse transcriptase (TERT) binds to and protects mtDNA from damage in mice. TERT binds at the coding regions of the ND1 and ND2 genes. TERT binding protects against ethidium bromide–induced damage and increases overall respiratory chain activity.39 In a recent study it was shown that TERT knockout mice exhibited a decrease in complex I activity, as well as reductions in complex II- and complex IV-dependent respiration.40 Meningiomas frequently show alterations in telomerase activity and exhibit a reduction of nucleus-encoded complex II, among others. We suggest that alterations to telomere function might contribute to the decline of OXPHOS enzyme expression in meningiomas.
As mentioned above, loss of chromosome 22q, which includes the NF2 gene, can be found in 85% of meningiomas.26 Almost all mutations of NF2 disrupt the function of merlin, the NF2 gene product.41 Most fascinating, merlin neutralizes the inhibitory effect of murine double minute on p53.42 In addition, it was shown that merlin may play a role in the regulation of mammalian target of rapamycin (mTOR) complex 1.43,44 Inhibition of mTOR enhances aerobic glycolysis and decreases mitochondrial respiration.45–47 Loss of merlin might therefore also lead to decreased OXPHOS.
With a proteomics approach, Sharma and colleagues identified several pathways affected in meningiomas, including mitochondrial function, OXPHOS, telomere extension, mTOR signaling, glycolysis, hypoxia, and vascular endothelial growth factor signaling. Numerous subunits of the OXPHOS complexes were reduced, depending on tumor grade. In addition, levels of some amino acids were altered in meningiomas.48,49
The fact that meningiomas show OXPHOS deficiencies may have therapeutic implications, since tumor cells depend on glucose for ATP production. The ketogenic diet is a high-fat, low-carbohydrate, energy-balanced diet that mimics the metabolic state of long-term fasting. During fasting or on a ketogenic diet, ketone bodies generated by the oxidation of fatty acids serve as fuel for the mitochondrial respiratory chain to cover the energy demands of normal body cells. By means of a ketogenic diet, it should be possible to target the increased glucose dependence of tumor cells to selectively starve them out. This should selectively slow down the proliferation and possibly kill cancer cells, whereas normal cells can use ketone bodies for energy production by OXPHOS. In case of meningiomas, ketogenic therapy might be applied when the tumor cannot be resected completely or is not operatively accessible. The different bioenergetic phenotypes of meningiomas probably influence the effect on tumor progression in individuals under a ketogenic diet as adjuvant therapy. In a few tumors of the central nervous system (astrocytic tumors, glioblastomas), it was shown that a calorie-restricted ketogenic diet had a growth inhibitory effect.50
Supplementary Material
Funding
This work was supported by the Salzburg Cancer Foundation, “Vereinigung zur Förderung der pädiatrischen Forschung und Fortbildung Salzburg,” the Children′s Cancer Foundation Salzburg, and the Austrian Research Promotion Agency (822782/THERAPEP).
Conflict of interest statement. The authors declare no competing financial interests.
Supplementary Material
References
- 1.Choy W, Kim W, Nagasawa D, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30(5):E6. [DOI] [PubMed] [Google Scholar]
- 2.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG, Huson SM, Donnai D, et al. A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992;29(12):841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5(12):1045–1054. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–353. [DOI] [PubMed] [Google Scholar]
- 7.Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–1653. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Gao P, Fukuda R, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–420. [DOI] [PubMed] [Google Scholar]
- 9.Meierhofer D, Mayr JA, Fink K, et al. Mitochondrial DNA mutations in renal cell carcinomas revealed no general impact on energy metabolism. Br J Cancer. 2006;94:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann FA, Mayr JA, Feichtinger R, et al. Respiratory chain complex I is a mitochondrial tumor suppressor of oncocytic tumors. Front Biosci. 2011;3:315–325. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann FA, Mayr JA, Neureiter D, et al. Lack of complex I is associated with oncocytic thyroid tumours. Br J Cancer. 2009;100(9):1434–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill AJ. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology. 2012;44(4):285–292. [DOI] [PubMed] [Google Scholar]
- 13.Feichtinger RG, Weis S, Mayr JA, et al. Alterations of oxidative phosphorylation complexes in astrocytomas. Glia. 2014;62(4):514–525. [DOI] [PubMed] [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feichtinger RG, Zimmermann F, Mayr JA, et al. Low aerobic mitochondrial energy metabolism in poorly- or undifferentiated neuroblastoma. BMC Cancer. 2010;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acham-Roschitz B, Plecko B, Lindbichler F, et al. A novel mutation of the RRM2B gene in an infant with early fatal encephalomyopathy, central hypomyelination, and tubulopathy. Mol Genet Metab. 2009;98(3):300–304. [DOI] [PubMed] [Google Scholar]
- 17.Cervos-Navarro J, Vazquez JJ. An electron microscopic study of meningiomas. Acta Neuropathol. 1969;13(4):301–323. [DOI] [PubMed] [Google Scholar]
- 18.Herting B, Meixensberger J, Roggendorf W, et al. Metabolic patterns in meningiomas. J Neurooncol. 2003;65(2):119–123. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Ray S, Mukherjee S, et al. Multipronged quantitative proteomic analyses indicate modulation of various signal transduction pathways in human meningiomas. Proteomics. 2015;15(2–3):394–407. [DOI] [PubMed] [Google Scholar]
- 20.Feichtinger RG, Neureiter D, Mayr JA, et al. Loss of mitochondria in ganglioneuromas. Front Biosci (Elite Ed). 2011;3:179–186. [DOI] [PubMed] [Google Scholar]
- 21.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19(8):1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz F, Fukui H, Garcia S, et al. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol. 2006;26(13):4872–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Fonzo A, Ronchi D, Lodi T, et al. The mitochondrial disulfide relay system protein GFER is mutated in autosomal-recessive myopathy with cataract and combined respiratory-chain deficiency. Am J Hum Genet. 2009;84(5):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeitink JA, Elpeleg O, Antonicka H, et al. Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am J Hum Genet. 2006;79(5):869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vahsen N, Cande C, Briere JJ, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23(23):4679–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Liu J, Patel S, et al. Genomic landscape of meningiomas. Brain Pathol. 2010;20(4):751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan Y, Hata N, Kuga D, et al. Narrowing of the regions of allelic losses of chromosome 1p36 in meningioma tissues by an improved SSCP analysis. Int J Cancer. 2008;122(8):1820–1826. [DOI] [PubMed] [Google Scholar]
- 28.Henn W, Cremerius U, Heide G, et al. Monosomy 1p is correlated with enhanced in vivo glucose metabolism in meningiomas. Cancer Genet Cytogenet. 1995;79(2):144–148. [DOI] [PubMed] [Google Scholar]
- 29.Lehtonen HJ, Makinen MJ, Kiuru M, et al. Increased HIF1 alpha in SDH and FH deficient tumors does not cause microsatellite instability. Int J Cancer. 2007;121(6):1386–1389. [DOI] [PubMed] [Google Scholar]
- 30.Kaynar MY, Sanus GZ, Hnimoglu H, et al. Expression of hypoxia inducible factor-1alpha in tumors of patients with glioblastoma multiforme and transitional meningioma. J Clin Neurosci. 2008;15(9):1036–1042. [DOI] [PubMed] [Google Scholar]
- 31.Yoo H, Baia GS, Smith JS, et al. Expression of the hypoxia marker carbonic anhydrase 9 is associated with anaplastic phenotypes in meningiomas. Clin Cancer Res. 2007;13(1):68–75. [DOI] [PubMed] [Google Scholar]
- 32.Ewald C, Hofmann T, Kuhn SA, et al. Methylation-specific multiplex ligation-dependent probe amplification in meningiomas. J Neurooncol. 2008;90(3):267–273. [DOI] [PubMed] [Google Scholar]
- 33.Favier J, Briere JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hervouet E, Demont J, Pecina P, et al. A new role for the von Hippel-Lindau tumor suppressor protein: stimulation of mitochondrial oxidative phosphorylation complex biogenesis. Carcinogenesis. 2005;26(3):531–539. [DOI] [PubMed] [Google Scholar]
- 35.Haase VH, Glickman JN, Socolovsky M, et al. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98(4):1583–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W, Sung HJ, Park JY, et al. A pivotal role for p53: balancing aerobic respiration and glycolysis. J Bioenerg Biomembr. 2007;39(3):243–246. [DOI] [PubMed] [Google Scholar]
- 37.Lebedeva MA, Eaton JS, Shadel GS. Loss of p53 causes mitochondrial DNA depletion and altered mitochondrial reactive oxygen species homeostasis. Biochim Biophys Acta. 2009;1787(5):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langford LA, Piatyszek MA, Xu R, et al. Telomerase activity in ordinary meningiomas predicts poor outcome. Hum Pathol. 1997;28(4):416–420. [DOI] [PubMed] [Google Scholar]
- 39.Haendeler J, Drose S, Buchner N, et al. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol. 2009;29(6):929–935. [DOI] [PubMed] [Google Scholar]
- 40.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470(7334):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueki K, Wen-Bin C, Narita Y, et al. Tight association of loss of merlin expression with loss of heterozygosity at chromosome 22q in sporadic meningiomas. Cancer Res. 1999;59(23):5995–5998. [PubMed] [Google Scholar]
- 42.Kim H, Kwak NJ, Lee JY, et al. Merlin neutralizes the inhibitory effect of Mdm2 on p53. J Biol Chem. 2004;279(9):7812–7818. [DOI] [PubMed] [Google Scholar]
- 43.James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29(15):4250–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragel BT, Jensen RL. Aberrant signaling pathways in meningiomas. J Neurooncol. 2010;99(3):315–324. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham JT, Rodgers JT, Arlow DH, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450(7170):736–740. [DOI] [PubMed] [Google Scholar]
- 46.Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci U S A. 2009;106(52):22229–22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schieke SM, Phillips D, McCoy JP, Jr, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281(37):27643–27652. [DOI] [PubMed] [Google Scholar]
- 48.Monleon D, Morales JM, Gonzalez-Segura A, et al. Metabolic aggressiveness in benign meningiomas with chromosomal instabilities. Cancer Res. 2010;70(21):8426–8434. [DOI] [PubMed] [Google Scholar]
- 49.Pfisterer WK, Hendricks WP, Scheck AC, et al. Fluorescent in situ hybridization and ex vivo 1H magnetic resonance spectroscopic examinations of meningioma tumor tissue: is it possible to identify a clinically-aggressive subset of benign meningiomas? Neurosurgery. 2007;61(5):1048–1059; discussion 1060–1061. [DOI] [PubMed] [Google Scholar]
- 50.Morscher RJ, Aminzadeh-Gohari S, Feichtinger RG, et al. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-Nu mouse model. PLOS One. 2015;10(6):e0129802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.