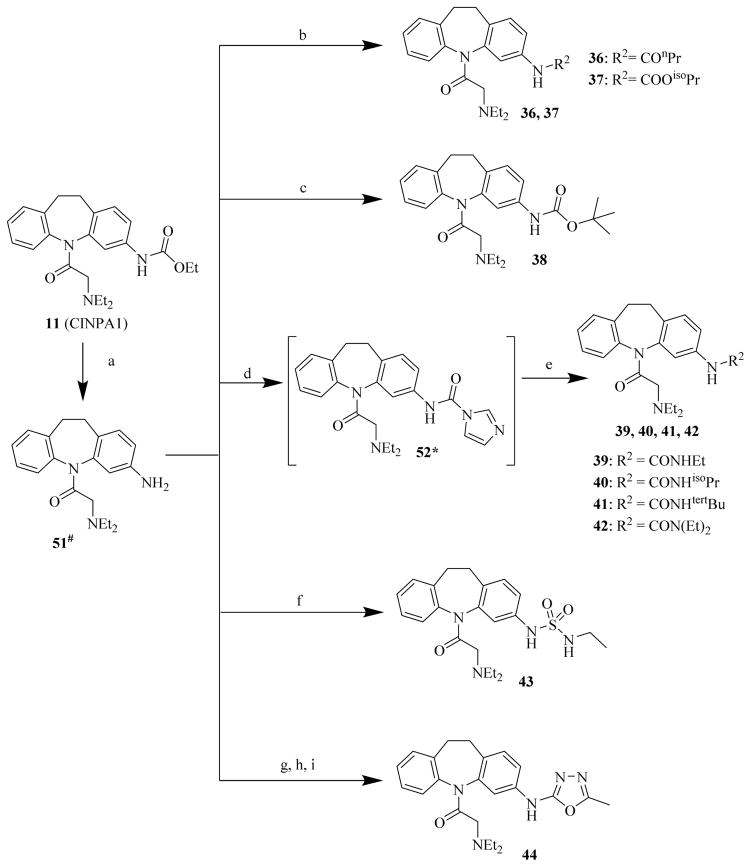
Scheme 4. Syntheses of CINPA1 analog compounds 36 to 44.
Reagents and conditions: (a) H2SO4, AcOH, 120°C, 4 h; (b): R2Cl, Et3N, dichloromethane, 10°C to 35°C, 16 h; (c): Boc2O, Et3N, dichloromethane, RT, 24 h; (d): CDI, Et3N, dichloromethane, RT, 2 h; (e): corresponding primary or secondary amine, DMAP, Et3N, dichloromethane, RT, 12 h; (f): EtNHSO2Cl, dichloromethane, Et3N, 15°C to 25°C, 1 h; (g): 1-(2-oxopyridine-1-carbothioyl)pyridin-2-one, dichloromethane, 20°C, 16 h; (h): acetohydrazide, THF, 20°C, 16 h; (i): EDC, Et3N, DMF, 20°C, 16 h.
#Purified or unpurified intermediate with partial structural characterization (1H NMR, MS, or both).
*This intermediate chemical was prepared (purified or unpurified) and used as raw starting material for the next step without structural characterization.