Abstract
Cell fate specification is a critical process to generate cells with a wide range of characteristics from stem and progenitor cells. Emerging evidence demonstrates that the orphan nuclear receptor COUP-TFII serves as a key regulator in determining the cell identity during embryonic development. The present review summarizes our current knowledge on molecular mechanisms by which COUP-TFII employs to define the cell fates, with special emphasis on cardiovascular and renal systems. These novel insights pave the road for future studies of regenerative medicine.
Keywords: COUP-TFII, heart, blood vessels, lymphatic vessels, kidney, cell fate specification
Introduction
Chicken Ovalbumin Upstream Promoter Transcription Factor II (COUP-TFII, also known as NR2F2) belongs to the steroid hormone receptor superfamily. COUP-TFII acts as a transcription factor to directly activate or repress transcriptional activities of target genes 1,2. Alternatively, COUP-TFII can sequester other transcription regulators, such as Smad4, TR, RXR and RAR, to affect the expression of downstream targets 2-4. At the cellular level, COUP-TFII promotes cell differentiation 5,6, proliferation 4,7,8, migration 7,8, survival 9 and intercellular communication 10-12. Analyses of genetically engineered mouse models reveal regulatory functions of COUP-TFII in the development of many organs and tissues, including cerebellum 13, brain 14, eye 15, heart 16-18, stomach 19, diaphragm 20, limb 21, kidney 9, adipose 22, testis 23 and blood and lymphatic vessels8,16,24,25. In adult physiology, COUP-TFII modulates male and female fertility 12,23,26 as well as glucose and energy metabolism 22. Pathologically, COUP-TFII facilitates tumor angiogenesis 10,27, promotes tumorigenesis 4 and suppresses endometriosis progression 28. Upstream regulators of COUP-TFII include retinoic acid 29,30, Sonic hedgehog 31-33, Indian hedgehog 34,35, cyclic AMP 30, IL-1β 28, TNFα 28, TGFβ1 28, Wnt/β-catenin 36, Sox7 37, Sox18 37, Notch 37,38 and microRNAs 28,39-41.
COUP-TFII’s relevance in human embryonic development finds support from numerous genetic studies. The human COUP-TFII genomic locus locates at 15q26 on chromosome 15. Mutations in the 15q26 region are shown to associate with congenital diaphragmatic hernia 42-44, analogous to the phenotype observed in COUP-TFII conditional knockout mice 20. In addition, patients with mutations in the COUP-TFII gene body and a subpopulation of patients with 15q26 mutations exhibit cardiac dysmorphogenesis 44-46. Interestingly, kidney abnormalities are also seen in many patients of 15q26 mutations who also had congenital heart defects and/or congenital diaphragmatic hernia 47.
In developing embryos, cell fate specification occurs when progenitor cells proceed to derive various types of terminally differentiated cells. During this process, genomic profiles change drastically to produce designated cellular phenotypes, which require coordinated control by networks of transcription factors and other regulatory mechanisms. COUP-TFII has been shown to be essential for specifying cell fates of fat, bone, muscle and eye progenitors 6. In the present article, we will summarize the role of COUP-TFII in cell fate decisions of cardiovascular and renal systems.
The Atrial Identity in Hearts
The atrial and ventricular cardiomyocytes exhibit differences in contractile properties, electric patterns, excitation-contraction coupling and endocrine functions, despite both of them serving as the main contractile apparatus in hearts 48,49. Morphologically, the atrial cardiomyocytes bear additional structural features as a hormone-producing cell with more extensively developed Golgi complexes, endoplasmic reticulum and storage granules 48,50. Gene expression studies further reveal distinct profiles between atria and ventricles that reflect functional and structural differences between the two compartments 51-53. These differences suggest unique regulatory programs may exist to confer chamber identities.
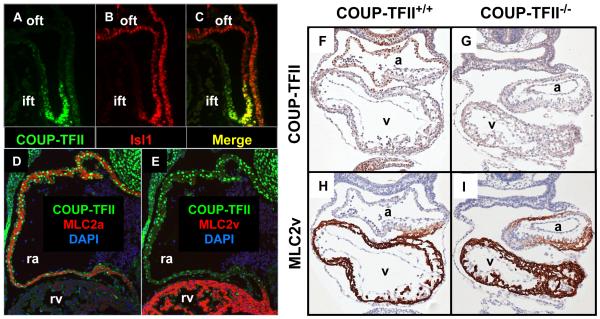
In developing human and mouse hearts, COUP-TFII is prominently expressed in the atria while its levels in ventricles are at the baseline level 16,51. At early stages of embryos, COUP-TFII expression is present in a subpopulation of Isl1+ progenitor cells at the posterior part of the second heart field near the venous pole of the heart tube (Figures 1A-C). Later on, the Isl1+ progenitor cells in this region will migrate into the heart tube and form the atrial compartment of chamber hearts 54-56. COUP-TFII continues to be present in cardiomyocytes of developing hearts and is co-localized with Myl7+ (MLC2a) atrial cardiomyocytes, but not with the Myl2+ (MLC2v) ventricular cells (Figures 1D-E). This expression pattern supports the hypothesis that COUP-TFII may contribute to specification of the atrial identity. Indeed, atria of COUP-TFII null mice adopt a ventricular phenotype, as evidenced by the ectopic expression of ventricular marker Myl2 in atria (Figures 1F-I). Further evidence indicates that maintaining the atrial identity requires COUP-TFII in immature cardiomyocytes 57. Deletion of COUP-TFII specifically in nascent cardiomyocytes reprograms atrial cardiomyocytes to be structurally, functionally and molecularly similar to ventricular cardiomyocytes in vivo 57. In contrast, ectopic COUP-TFII overexpression is also sufficient to confer the atrial phenotype to immature ventricular cardiomyocytes 57. Notably, the COUP-TFII dependent plasticity of chamber identity is transient because such an identity switch is no longer seen when COUP-TFII is deleted at embryonic day 15 57. Collectively, these findings demonstrate that COUP-TFII serves as an important molecular regulator in specification of the atrial identity.
Figure 1. COUP-TFII expression patterns in developing hearts and the cardiac phenotype in COUP-TFII null mice.
(A-C) Cardiogenic area in sagittal sections of 27-somite wild type embryos stained for COUP-TFII (green) and cardiac progenitor marker Isl1 (red). (C) The merged image of (A) and (B). (D and E) Cross sections of E11.5 wild type embryos stained for denoted markers. DAPI marks nuclei. (F-I) Immunostaining of embryos at the 23-somite stage. Hematoxylin in blue serves as nuclear counterstaining. (F and H) Wild type mice, (G and I) COUP-TFII null mice, (F and G) COUP-TFII stained in brown and (H and I) Ventricular marker MLC2v stained in brown. Ift, inflow tract; oft, outflow tract; ra, right atrium; rv, right ventricle; a, primitive atrium; v, primitive ventricle.
COUP-TFII employs a network of transcription factors, including Tbx5, Hey2, and Irx4 to specify the atrial identity. Tbx5 promotes atrial gene expression and is essential for atrial morphogenesis 58,59. COUP-TFII binds at the Tbx5 genomic locus and positively modulates Tbx5 expression, suggesting that Tbx5 is a direct downstream target of COUP-TFII 57. COUP-TFII may control Tbx5 transcription through interaction with Sp1 because a Sp1 binding site is required for COUP-TFII dependent promotion of Tbx5 expression 57. The ventricular transcription factors Hey2 and Irx4 are necessary and sufficient to suppress expression of atrial genes 58,60-64. It was further demonstrated that COUP-TFII silences the expression of Hey2 and Irx4 in atria through direct binding to imperfect direct repeat sequences of AGGTCA in Hey2 and Irx4 genomic loci and suppresses the expression of both genes 57. Aside from controlling the expression of major transcription regulators, COUP-TFII also directly regulates a broad spectrum of genes that are important for atrial development and function. In embryonic atrial tissues, chromatin immunoprecipitation and sequencing assays (ChIP-seq) identified more than two thousand COUP-TFII binding sites that could be potential enhancers/repressors for a wide spectrum of cardiac genes. For example, COUP-TFII binds at and modulates expression of contractile genes Myl2, Myl7 and Myl4, ion channel genes Kcne1, Kcng2, Kcnj5, Kcnk2, Cacna1c and Cacna1d, growth factors Fgf1 and Fgf12, and cardiac transcription factors Lbh and Id2 57. Taken together, these findings support that specification of the atrial identity requires a COUP-TFII dependent regulatory network, in which COUP-TFII controls expression of a cascade of transcriptional and physiological regulators as well as cell type specific genes.
The COUP-TFII dependent specification of cardiomyocyte fate is also seen in fruit flies. The Drosophila COUP-TF homolog, seven-up, specifies a subpopulation of cardiac precursor cells fated for the future ostia, while tinman, homolog of vertebrate Nkx homeobox gene and a master cardiac transcription factor, marks the rest of the cardiac precursors that give rise to other segments of the heart 65,66. Functionally, seven-up is essential and sufficient to repress the expression tinman for specification of tinman-negative cardiac precursors 65. Interestingly, zebrafish require Nkx2.5 and Nkx2.7 to maintain their ventricular identity. Loss of Nkx2.5/Nkx2.7 results in adoption of atrial identity by ventricular cardiomyocytes 67. Collectively, these findings implicate a potential role of Nkx homeobox transcription factors in the COUP-TFII dependent regulatory network for cardiac fate determination.
The Venous and Lymphatic Fate Specification in Vasculature
Arterial, venous and lymphatic vessels are the three types of major conduits to circulate body fluid. Blood pumped out from cardiac ventricles travels through arteries to reach micro vessels in peripheral tissue and returns via veins to atria. Meanwhile, lymphatic vessels collect and return interstitial fluid to the general circulation via connecting to subclavian veins. Fates of arteries and veins are determined during embryonic development 68. We and others show that COUP-TFII and Notch signaling serve as key regulators in specification of venous and arterial fates, respectively 25,69-72. Artery-fated angioblasts receive signals from vascular endothelial growth factor (VEGF) via the receptor VEGFR2 and the co-receptor neuropilin 1 (NRP1) to activate Notch signaling for further differentiation toward the arterial phenotype. Additionally, forkhead transcription factors Foxc1 and Foxc2 also promote arterial gene expression through increasing expression of Notch signaling genes Dll4 and Hey2 73,74. In contrast, vein-fated angioblasts express COUP-TFII, which suppresses notch signaling to confer vein identity. COUP-TFII directly suppresses expression of NRP1 and FOXC1, two upstream regulators of Notch signaling 7. The resulting suppression of the Notch signaling is evidenced by reduced expression of Notch downstream effectors HES1, HES2 and HEY2, increased levels of venous marker gene EPHB4, and decreased expression of arterial marker EFNB2. Additionally, COUP-TFII also binds to the promoter of the HEY2 gene and directly represses its transcriptional activities 7. Thus, COUP-TFII specifies the venous fate through suppression of the Notch signaling pathway at multiple points. Notably, Notch is shown to repress COUP-TFII expression in the dorsal aorta of zebrafish and cultured endothelial cells 37,38, suggesting a reciprocal repression mechanism of COUP-TFII and Notch during vascular development.
Recently, the chromatin remodeling enzyme Brg1 (also known as Smarca4) has been shown to positively regulate COUP-TFII expression, and is required for venous specification in mouse embryos 75. Brg1 binds at the promoter and a −1.2-Kb upstream region of COUP-TFII gene. Brg1 depletion results in a reduced H3K9ac active enhancer mark and increased histone H3 binding at both Brg1 binding sites, leading to decreased RNA polymerase II recruitment at the COUP-TFII promoter region and reduced COUPTFII expression 75. Veins of endothelial-specific Brg1 knockout mice exhibit decreased COUP-TFII expression and increased NRP1 and DLL4 levels, parallel to the COUP-TFII knockout phenotype 75. Collectively, these findings indicate that Brg1 suppresses Notch signaling through epigenetically modifying COUP-TFII gene activities for venous fate specification.
The lymphatic vessels arise from the anterior cardinal veins and intersomitic vessels 76,77. In mid-gestation embryos, a subset of endothelial cells fated as lymphatic precursors migrate out of blood vessels to form lymphatic sac and subsequent lymphatic vasculature76-78. Specification of lymphatic precursors requires COUP-TFII and Sox18 to promote expression of the lymphatic master regulator Prox1, in which direct transcriptional regulation serves as the underlying mechanism of action 24,79. In addition, ERK signaling also stimulates Sox18 and Prox1 expression for lymphatic specification 80. The ERK signaling may work in parallel or downstream of COUP-TFII because constitutive activation of ERK does not affect COUP-TFII expression 80. After fate specification, the Prox1-positive lymphatic precursors bud out and leave blood vessels to undergo further differentiation and sprouting in response to mesenchyme-derived vascular endothelial growth factor C (VEGFC) 77. In lymphatic precursors, expression of the VEGFC receptor, VEGFR3, requires physical interaction between COUP-TFII and Prox1 to jointly promote its transcription 81. Regulation of VEGFR3 by COUP-TFII continues to be essential during the process of lymphangiogenesis. Loss of COUP-TFII results in decreased VEGFR3 expression and impairs proliferation and migration of lymphatic endothelial cells 8. In summary, COUP-TFII controls lymphatic system development by regulating cell fate specification, migration, proliferation and differentiation.
Fate Determination of Renal Progenitor Cells
During embryogenesis, kidneys derive from renal progenitor cells in the metanephric mesenchyme. The metanephric mesenchyme arises from a subpopulation of intermediate mesodermal cells that are specified to adopt the renal progenitor fate 82. The intermediate mesodermal cells express high levels of COUP-TFII and COUP-TFII deficient mice fail to develop metanephric mesenchyme 9. These findings together indicate that specification of renal progenitors requires COUP-TFII. After formation of metanephric mesenchyme, COUP-TFII remains prominently expressed in these renal progenitors. At this stage, COUP-TFII works in parallel with Osr1, another master renal development gene, to regulate the Eya1-Pax2-Six2 axis that promotes expression of Gdnf for subsequent induction of nephron formation 83. At the same time, COUP-TFII also promotes expression of the Wt1 gene that is essential for survival of renal progenitors cells in the metanephric mesenchyme. Mechanistically, COUP-TFII modulates these two pathways by Sp1-dependent binding at the Eya1 and Wt1 promoters to induce their transcriptional activities 9. Taken together, COUP-TFII is central to a network of renal transcriptional regulators that control fate specification, cell survival and intercellular communication of renal progenitors.
The nephron is the working unit of a kidney. Each nephron is segmented into four functional domains – glomerulus, proximal tubule, loop of Henle and distal tubule. During the process of nephron segmentation, Notch2 specifies the identity of proximal tubules. Deletion of Notch2 results in kidney hypoplasia where glomeruli and proximal tubules are absent but the distal tubules remain in place 84. On the other hand, COUPTFII is expressed in distal tubules but not in proximal tubules of developing kidneys 85,86. Interestingly, deletion of COUP-TFII in renal progenitor cells (CKOsix2-cre) also produces kidney hypoplasia with an opposite phenotype in which proximal tubules form with much less corresponding distal tubules (Yu et al., unpublished observations). Further analysis found that the COUP-TFII deficient tubule cells acquire the capacity to bind Lotus tetragonolobus lectin (LTL) that marks the proximal tubules 86, suggesting that cells alter characters subsequent to loss of COUP-TFII, possibly through adaptation of the proximal tubule fate. These findings collectively implicate that, in contrast to Notch2 dependent specification of proximal tubule fate, COUP-TFII may play a critical role in specifying cells that are fated for distal tubules during nephron segmentation.
In adult kidney, COUP-TFII is required to maintain expression of renin 87, the enzyme that activates the renin-angiotensin system to regulate blood pressure. COUPTFII and renin are both expressed in the juxtaglomerular cells that are the primary renin-expression cells 87. Deletion of COUP-TFII, after the kidney is fully matured, reduces the basal levels of renin expression in renin-expressing cells. At the molecular level, COUPTFII binds to a direct repeat at the proximal renin promoter and works with another transcription factor cAMP-binding protein to promote renin expression 87. It is worthy to note that genome-wide association studies have linked COUP-TFII to hypertension in human 88,89, supporting the clinical relevance of COUP-TFII in regulation of the reninangiotensin system. Intriguingly, the renin expressing cells also arise from progenitors in the metanephric mesenchyme where COUP-TFII is highly expressed 9, while emerging evidence suggests that Notch signaling regulates the expansion of renin-expressing cell population under pathological conditions through cellular reprogramming 90-92. Owing to the close relation between COUP-TFII and Notch signaling in cell fate determination, it is tempting to speculate that COUP-TFII might also participate in this reprogramming event at the adult stage.
Conclusions and Remarks
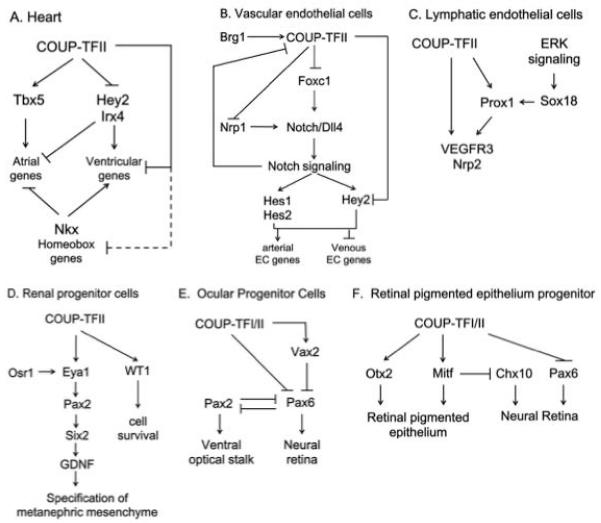
COUP-TFII serves as a master regulator for cell fate specification in multiple tissues. Numerous studies indicate that COUP-TFII establishes specific cell identities in conjunction with or working through various key transcriptional regulators (Figure 2). Although compositions of COUP-TFII dependent regulatory networks may be context-dependent, control of common important cell fate determinants by COUP-TFII are conserved among tissues. For example, in hearts and blood vessels, COUP-TFII directly suppresses transcription of the Hey2 gene, which is a common regulator for ventricular and arterial fate specification 7,57. Furthermore, COUP-TFs repress Pax2 gene expression in both renal and ocular progenitors, despite going through different effectors9,15. On the other hand, collaborations between COUP-TFII and other transcription regulators may increase COUP-TFII’s functional diversity. This mechanism has been shown to result in distinct genomic profiles that underlie individual cell fates 93. Therefore, genome-wide motif analyses in COUP-TFII binding regions and unbiased proteomic analyses of COUP-TFII interacting proteins should facilitate the discovery of COUP-TFII dependent genetic codes that are utilized to determine cell fates. Interestingly, deletion of COUPTFII at the adult stage does not produce overt phenotypes, which suggests that COUPTFII may mainly operate at a permissive window of plasticity for cell fate specification 57. This notion finds further support in previous studies that fate plasticity is time-sensitive in hearts and blood vessels 94,95. With the emerging cell-based therapeutic strategies in sight, learning from COUP-TFII dependent regulatory mechanisms would help to better understand the time and molecular circuits that define cell fates.
Figure 2.
Summary of COUP-TFII dependent regulatory networks in cardiovascular and renal systems.
Highlights.
COUP-TFII is expressed in progenitor cells of multiple lineages.

COUP-TFII controls major regulators of cell fate specification.

This review focuses on the role of COUP-TFII in cardiovascular and renal systems.
Acknowledgments
This work was supported by grants from NIH DK59820 and HL114539 (S.Y.T. and M.J.T.) and DK45641 (M.J.T.). We thank Mrs. Jodie R. Hebert for manuscript editing. The authors acknowledge the joint participation by Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine and the Cancer Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Achatz G, et al. Functional domains of the human orphan receptor ARP-1/COUP-TFII involved in active repression and transrepression. Mol Cell Biol. 1997;17:4914–32. doi: 10.1128/mcb.17.9.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev. 1997;18:229–40. doi: 10.1210/edrv.18.2.0294. [DOI] [PubMed] [Google Scholar]
- 3.Butler AJ, Parker MG. COUP-TF II homodimers are formed in preference to heterodimers with RXR alpha or TR beta in intact cells. Nucleic Acids Res. 1995;23:4143–50. doi: 10.1093/nar/23.20.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin J, et al. COUP-TFII inhibits TGF-beta-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–40. doi: 10.1038/nature11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X, Qin J, Lin SH, Tsai SY, Tsai MJ. Nuclear receptor chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) modulates mesenchymal cell commitment and differentiation. Proc Natl Acad Sci U S A. 2011;108:14843–8. doi: 10.1073/pnas.1110236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, Tang K, Yu CT, Tsai SY, Tsai MJ. Regulatory potential of COUPTFs in development: stem/progenitor cells. Semin Cell Dev Biol. 2013;24:687–93. doi: 10.1016/j.semcdb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Qin J, Cheng CM, Tsai MJ, Tsai SY. COUP-TFII Is a Major Regulator of Cell Cycle and Notch Signaling Pathways. Mol Endocrinol. 2012;26:1268–77. doi: 10.1210/me.2011-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FJ, et al. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120:1694–707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CT, et al. COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival. Development. 2012;139:2330–9. doi: 10.1242/dev.076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Chen X, Yu-Lee LY, Tsai MJ, Tsai SY. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res. 2010;70:8812–21. doi: 10.1158/0008-5472.CAN-10-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurihara I, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DK, et al. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–40. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BJ, Takamoto N, Yan J, Tsai SY, Tsai MJ. Chicken Ovalbumin Upstream Promoter-Transcription Factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol. 2009;326:378–91. doi: 10.1016/j.ydbio.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang K, Rubenstein JLR, Tsai SY, Tsai M-J. COUP-TFII controls amygdala patterning by regulating neuropilin expression. Development. 2012;139:1630–1639. doi: 10.1242/dev.075564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang K, et al. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development. 2010;137:725–34. doi: 10.1242/dev.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin FJ, et al. Endocardial cushion morphogenesis and coronary vessel development require chicken ovalbumin upstream promoter-transcription factor II. Arterioscler Thromb Vasc Biol. 2012;32:e135–46. doi: 10.1161/ATVBAHA.112.300255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–49. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu SP, Lee DK, Demayo FJ, Tsai SY, Tsai MJ. Generation of ES cells for conditional expression of nuclear receptors and coregulators in vivo. Mol Endocrinol. 2010;24:1297–304. doi: 10.1210/me.2010-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takamoto N, et al. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development. 2005;132:2179–89. doi: 10.1242/dev.01808. [DOI] [PubMed] [Google Scholar]
- 20.You LR, et al. Mouse lacking COUP-TFII as an animal model of Bochdalektype congenital diaphragmatic hernia. Proc Natl Acad Sci U S A. 2005;102:16351–6. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CT, et al. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol. 2004;24:10835–43. doi: 10.1128/MCB.24.24.10835-10843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, et al. The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab. 2009;9:77–87. doi: 10.1016/j.cmet.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan RS, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You LR, et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 26.Takamoto N, et al. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19:2299–308. doi: 10.1210/me.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci U S A. 2010;107:3687–92. doi: 10.1073/pnas.0914619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SC, et al. Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2014;99:E427–37. doi: 10.1210/jc.2013-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruse SW, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soosaar A, Neuman K, Nornes HO, Neuman T. Cell type specific regulation of COUP-TF II promoter activity. FEBS Lett. 1996;391:95–100. doi: 10.1016/0014-5793(96)00711-9. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan V, Elberg G, Tsai MJ, Tsai SY. Identification of a novel sonic hedgehog response element in the chicken ovalbumin upstream promotertranscription factor II promoter. Mol Endocrinol. 1997;11:1458–66. doi: 10.1210/mend.11.10.9992. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan V, et al. Mediation of Sonic hedgehog-induced expression of COUPTFII by a protein phosphatase. Science. 1997;278:1947–50. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, et al. Sonic hedgehog (Shh) regulates the expression of angiogenic growth factors in oxygen-glucose-deprived astrocytes by mediating the nuclear receptor NR2F2. Mol Neurobiol. 2013;47:967–75. doi: 10.1007/s12035-013-8395-9. [DOI] [PubMed] [Google Scholar]
- 34.Lee K, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–9. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 35.Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–48. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- 36.Okamura M, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A. 2009;106:5819–24. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swift MR, et al. SoxF factors and Notch regulate nr2f2 gene expression during venous differentiation in zebrafish. Dev Biol. 2014;390:116–25. doi: 10.1016/j.ydbio.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang J, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–50. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu S, et al. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells. 2013;31:259–68. doi: 10.1002/stem.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong BC, Kang IH, Hwang YC, Kim SH, Koh JT. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014;5:e1532. doi: 10.1038/cddis.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang IH, et al. MicroRNA-302a stimulates osteoblastic differentiation by repressing COUP-TFII expression. J Cell Physiol. 2015;230:911–21. doi: 10.1002/jcp.24822. [DOI] [PubMed] [Google Scholar]
- 42.Brady PD, et al. Identification of dosage-sensitive genes in fetuses referred with severe isolated congenital diaphragmatic hernia. Prenat Diagn. 2013;33:1283–92. doi: 10.1002/pd.4244. [DOI] [PubMed] [Google Scholar]
- 43.Klaassens M, et al. Prenatal detection and outcome of congenital diaphragmatic hernia (CDH) associated with deletion of chromosome 15q26: two patients and review of the literature. Am J Med Genet A. 2007;143A:2204–12. doi: 10.1002/ajmg.a.31892. [DOI] [PubMed] [Google Scholar]
- 44.Lopez I, et al. Prenatal diagnosis of de novo deletions of 8p23.1 or 15q26.1 in two fetuses with diaphragmatic hernia and congenital heart defects. Prenat Diagn. 2006;26:577–80. doi: 10.1002/pd.1468. [DOI] [PubMed] [Google Scholar]
- 45.Al Turki S, et al. Rare variants in NR2F2 cause congenital heart defects in humans. Am J Hum Genet. 2014;94:574–85. doi: 10.1016/j.ajhg.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorsson T, et al. Chromosomal Imbalances in Patients with Congenital Cardiac Defects: A Meta-analysis Reveals Novel Potential Critical Regions Involved in Heart Development. Congenit Heart Dis. 2014 doi: 10.1111/chd.12179. [DOI] [PubMed] [Google Scholar]
- 47.Lurie IW. Kidney abnormalities in persons with monosomy 15q26. Am J Med Genet A. 2008;146A:1761–4. doi: 10.1002/ajmg.a.32333. [DOI] [PubMed] [Google Scholar]
- 48.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 49.Ng SY, Wong CK, Tsang SY. Differential gene expressions in atrial and ventricular myocytes: insights into the road of applying embryonic stem cell-derived cardiomyocytes for future therapies. Am J Physiol Cell Physiol. 2010;299:C1234–49. doi: 10.1152/ajpcell.00402.2009. [DOI] [PubMed] [Google Scholar]
- 50.Jamieson JD, Palade GE. Specific Granules in Atrial Muscle Cells. J Cell Biol. 1964;23:151–72. doi: 10.1083/jcb.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barth AS, et al. Functional profiling of human atrial and ventricular gene expression. Pflugers Arch. 2005;450:201–8. doi: 10.1007/s00424-005-1404-8. [DOI] [PubMed] [Google Scholar]
- 52.McGrath MF, de Bold AJ. Transcriptional analysis of the mammalian heart with special reference to its endocrine function. BMC Genomics. 2009;10:254. doi: 10.1186/1471-2164-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabibiazar R, Wagner RA, Liao A, Quertermous T. Transcriptional profiling of the heart reveals chamber-specific gene expression patterns. Circ Res. 2003;93:1193–201. doi: 10.1161/01.RES.0000103171.42654.DD. [DOI] [PubMed] [Google Scholar]
- 54.Cai CL, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominguez JN, Meilhac SM, Bland YS, Buckingham ME, Brown NA. Asymmetric fate of the posterior part of the second heart field results in unexpected left/right contributions to both poles of the heart. Circ Res. 2012;111:1323–35. doi: 10.1161/CIRCRESAHA.112.271247. [DOI] [PubMed] [Google Scholar]
- 56.Meilhac SM, Lescroart F, Blanpain C, Buckingham ME. Cardiac cell lineages that form the heart. Cold Spring Harb Perspect Med. 2014;4:a013888. doi: 10.1101/cshperspect.a013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu S.-p., et al. Atrial Identity Is Determined by a COUP-TFII Regulatory Network. Developmental cell. 2013;25:417–426. doi: 10.1016/j.devcel.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruneau BG, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–21. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 59.Mori AD, et al. Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev Biol. 2006;297:566–86. doi: 10.1016/j.ydbio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 60.Bao ZZ, Bruneau BG, Seidman JG, Seidman CE, Cepko CL. Regulation of chamber-specific gene expression in the developing heart by Irx4. Science. 1999;283:1161–4. doi: 10.1126/science.283.5405.1161. [DOI] [PubMed] [Google Scholar]
- 61.Fischer A, et al. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol Cell Biol. 2005;25:8960–70. doi: 10.1128/MCB.25.20.8960-8970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koibuchi N, Chin MT. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res. 2007;100:850–5. doi: 10.1161/01.RES.0000261693.13269.bf. [DOI] [PubMed] [Google Scholar]
- 63.Xin M, et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci U S A. 2007;104:7975–80. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- 65.Lo PC, Frasch M. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech Dev. 2001;104:49–60. doi: 10.1016/s0925-4773(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 66.Reim I, Mohler JP, Frasch M. Tbx20-related genes, mid and H15, are required for tinman expression, proper patterning, and normal differentiation of cardioblasts in Drosophila. Mech Dev. 2005;122:1056–69. doi: 10.1016/j.mod.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Targoff KL, et al. Nkx genes are essential for maintenance of ventricular identity. Development. 2013;140:4203–13. doi: 10.1242/dev.095562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–9. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 69.Duarte A, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–8. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol. 2005;278:301–9. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 71.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 72.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–20. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seo S, et al. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–70. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 75.Davis RB, Curtis CD, Griffin CT. BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Development. 2013;140:1272–81. doi: 10.1242/dev.087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srinivasan RS, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–32. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–8. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oliver G, Srinivasan RS. Lymphatic vasculature development: current concepts. Ann N Y Acad Sci. 2008;1131:75–81. doi: 10.1196/annals.1413.006. [DOI] [PubMed] [Google Scholar]
- 79.Francois M, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–7. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 80.Deng Y, Atri D, Eichmann A, Simons M. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest. 2013;123:1202–15. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S, et al. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–9. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyle S, de Caestecker M. Role of transcriptional networks in coordinating early events during kidney development. Am J Physiol Renal Physiol. 2006;291:F1–8. doi: 10.1152/ajprenal.00447.2005. [DOI] [PubMed] [Google Scholar]
- 83.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 84.Cheng HT, et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–11. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jonk LJ, et al. Cloning and expression during development of three murine members of the COUP family of nuclear orphan receptors. Mech Dev. 1994;47:81–97. doi: 10.1016/0925-4773(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 86.Suh JM, et al. The expression profiles of nuclear receptors in the developing and adult kidney. Mol Endocrinol. 2006;20:3412–20. doi: 10.1210/me.2006-0312. [DOI] [PubMed] [Google Scholar]
- 87.Mayer S, et al. Chicken ovalbumin upstream promoter transcription factor II regulates renin gene expression. J Biol Chem. 2012;287:24483–91. doi: 10.1074/jbc.M111.329474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Browning BL, Browning SR. Haplotypic analysis of Wellcome Trust Case Control Consortium data. Hum Genet. 2008;123:273–80. doi: 10.1007/s00439-008-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wellcome Trust Case Control, C. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castellanos Rivera RM, et al. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–8. doi: 10.1152/physiolgenomics.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–28. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 92.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol. 2001;281:F345–56. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- 93.Aranguren XL, et al. COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or hetero-dimerisation with PROX1. J Cell Sci. 2013;126:1164–75. doi: 10.1242/jcs.116293. [DOI] [PubMed] [Google Scholar]
- 94.Gruber PJ, Kubalak SW, Chien KR. Downregulation of atrial markers during cardiac chamber morphogenesis is irreversible in murine embryos. Development. 1998;125:4427–38. doi: 10.1242/dev.125.22.4427. [DOI] [PubMed] [Google Scholar]
- 95.Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–70. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]