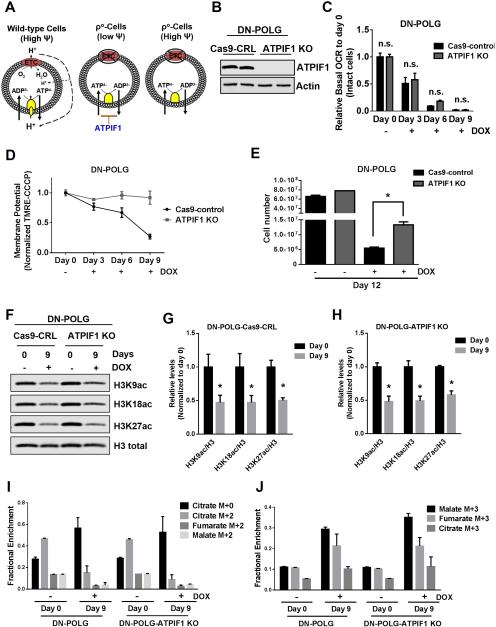
Figure 6. Restoration of the mitochondrial membrane potential in inducible mtDNA depleted cells partially rescues cell proliferation but not specific histone 3 acetylation marks.
(A) A functional ETC generates a mitochondrial membrane potential that is used to produce ATP. In ρ° cells, an impaired ETC is not able to generate a mitochondrial membrane potential. ρ° cells are able to sustain mitochondrial membrane potential through the electrogenic exchange of ATP4- for ADP3- by the adenine nucleotide carrier. This mechanism relies on the F1-ATPase activity of an incomplete F0F1-ATPase that is loosely associated with the membrane. However, the ATPIF1 protein, an inhibitor of the F1-ATPase prevents the maintenance of the mitochondrial membrane potential. The loss of ATPIF1 increases the F1-ATPase activity to sustain the mitochondrial membrane potential.
(B) Western blot analysis of ATPIF1 protein levels in DN-POLG-Cas9-control cells and DN-POLG-ATPIF1 KO cells.
(C-D) Relative mitochondrial oxygen consumption rate (OCR) (C) and mitochondrial membrane potential (D) of intact DN-POLG-Cas9-control cells and DN-POLG-ATPIF1 KO cells untreated or treated with doxycycline (10 ng/ml) for 3, 6 and 9 day. Mean ± SEM (n=3).
(E) DN-POLG-Cas9-control cells and DN-POLG-ATPIF1 KO cells were grown in the presence or absence of doxycycline (10 ng/ml) for 12 days and assessed for cell number. Mean ± SEM (n=3).
(F-H) Western blot analysis and quantification of the acetylation of H3K9, H3K18 and H3K27 and the total expression of H3 in DN-POLG-Cas9-nt-control and DN-POLG-ATPIF1 KO cells untreated or treated with doxycycline (10 ng/ml) for 9 days. Data was normalized to the mean value in untreated cells. Mean ± SEM (n=3).
(I-J) DN-POLG and DN-POLG-ATPIF1 KO cells untreated or treated with doxycycline (10 ng/ml) for 9 days were labeled for six hours with [U-13C]glucose and subsequently metabolite pools were examined. m+0 pools represent unlabeled fractions. m+2 and m+3 pools results from glucose flow through pyruvate dehydrogenase and pyruvate carboxylase, respectively. Mean ± SEM (n=3).
* Indicates significance p < 0.05 throughout figure 6. See also Figure S6.