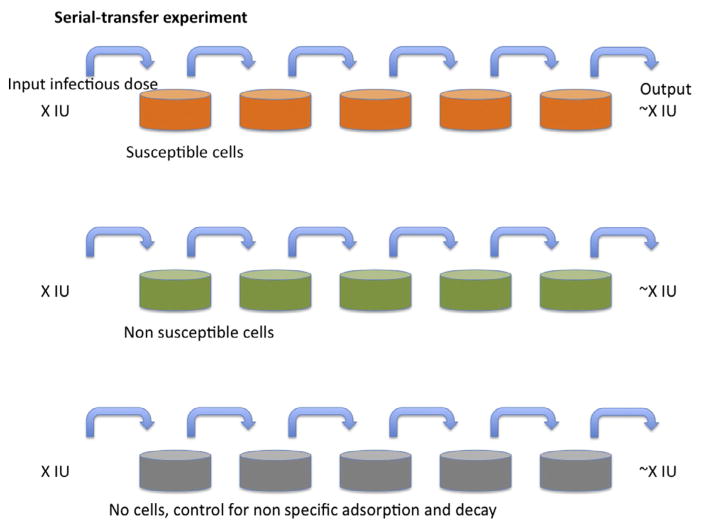
Figure 1. Serial transfer.
A nominal infectious dose (X IU) of virus is added to the tissue-culture dishes in the left-hand column. After incubation for a fixed number of hours and at a constant temperature, the medium is aspirated from the second dish, to which all of the medium is transferred from the first dish, which is replenished with medium without virus. The procedure is repeated until the right-most dish has been incubated with supernatant for the same time as the others. The top row of dishes (orange (gray in the print version)) have susceptible cells forming a nonconfluent cell matt. The middle row (green (gray in the print version)) have nonsusceptible but otherwise similar cells (for example, lacking only a crucial receptor) growing at the same confluency. The bottom row has only tissue-culture dishes without cells. The outcome of the classical experiment as outlined is usually that infectious virus is transferred from the left to the right at nearly constant levels as detected in each dish; or there may be nonspecific losses, for example, some virus is lost for each step also in the middle row by binding to cell-surface glycosaminoglycans, equally abundant on nonsusceptible cells; or the non-specific losses through binding only to plastic (bottom row) may be equally great; or the infectivity declines significantly in the medium within the time-frame of the experiment (not shown). The key finding is that the losses attributable to infection are negligible and that is because the diffusion time from the top to the bottom of the medium in the tissue-culture dish is too long for more than a small fraction of the virions to encounter target cells through diffusion.