Figure 7.
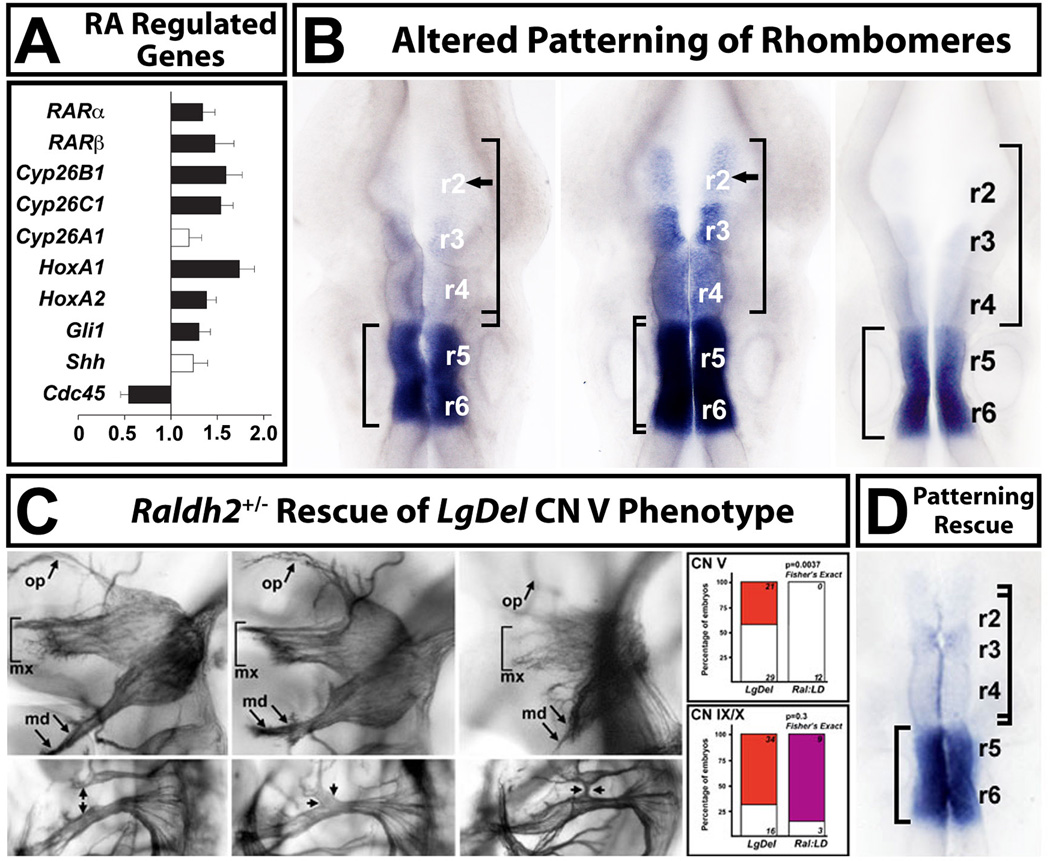
Retinoic acid (RA) signaling is altered in the LgDel hindbrain, leading to changes in anterior-posterior patterning. A. qPCR analysis, in microdissected E9.5 hindbrains from WT and LgDel embryos, of a subset of highly RA-regulated genes known to be expressed at substantial levels in the E9.5 hindbrain, as well as two control genes (Shh, and Cdc45, a 22q11 gene). Expression levels for all but one of these genes (Cyp26a1) are significantly increased in the E9.5 hindbrain. B. Disrupted pattern of RA-regulated gene expression in the E9.5 hindbrain of LgDel, but not Tbx1+/− embryos. We used in situ hybridization (ISH) for Cyp26b1, which is highly retinoid regulated in WT, and has a distinct posterior-hindbrain expression pattern: enhanced in r5 and r6 with diminished expression in r3 and r4 and little to no expression in r2 (left). There is a dramatic anterior shift of expression, in parallel with the increased expression seen in our qPCR measurements (see panel A), in the LgDel embryo (middle). There is now substantial Cyp26b1 expression in r3 and r4, and expression continues into r2. This shift is consistent with a “posteriorizing” influence of altered RA signaling on hindbrain differentiation in the LgDel. A similar expansion of Cyb26b1 labeling is not seen in the Tbx1+/− hindbrain (right). C. Lowering RA signaling levels in LgDel mice by approximately 25% [103] using a heterozygous null allele of the RA synthetic enzyme Raldh2, results in rescue of the CN V phenotypes in LgDel, but not CN IX and X phenotypes. The CN V rescue in LgDel was remarkably statistically robust (far right, top); however there is no statistical evidence for rescue of the CN IX/X phenotype (far right, bottom). D. ISH analysis of Cyp26b1 expression in Raldh2+/−;LgDel compound embryos shows that the expression levels and pattern of this RA-regulated gene are now comparable to WT. qPCR analysis (not shown; Karpinski et al, 2014) is consistent with this rescue; RA dependent genes are restored in their expression to levels indistinguishable from WT. Figure adapted from (Karpinski et al., 2014).