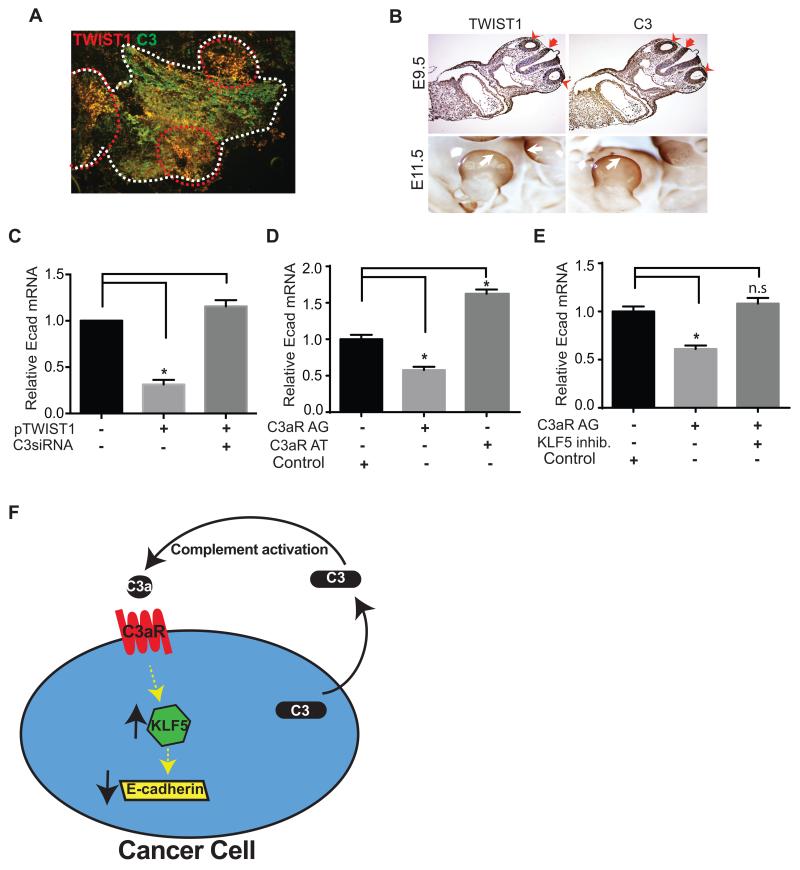
FIGURE 4. Coexpression of C3 and TWIST1 in implanted tumors in mice and in developing murine embryos.
(A) Expression of TWIST1 and C3 in implanted ovarian tumor tissue was analyzed using immunofluorescence microscopy. ID8-VEGF cells-induced tumors were resected and immunostained with TWIST1 (red) and C3 (green) antibodies. Borders of a tumor nodule are shown using white broken lines. Co-expression of TWIST1 and C3 (yellow) was more prominent at the invasive edges of the tumor (circumscribed with red broken lines). (B) Mouse embryos were collected at 9.5dpc (upper panels) and 11.5dpc (whole mounted, lower panels), and immunohistochemistry was performed using anti-TWIST1 or anti-C3 antibody. TWIST1 and C3 were co-localized in the otocysts (red arrow head) and hindbrain (red arrow) at 9.5dpc embryo, and in the limb-bud (white arrow) at 11.5dpc embryos. (C) To study the effect of C3 on the TWIST1-mediated gene regulation, E-cadherin mRNA level was measured before and after transient transfection of C3 siRNA into TWIST1-overexpressing HeyA8 cells (stably transfected with plasmid encoding TWIST1 cDNA). Scrambled siRNAs were used as negative controls. TWIST1 overexpression was associated with 70% decrease in E-cadherin mRNA level (n=3, *p=0.0002). C3 knockdown restored E-cadherin expression in TWIST1 overexpressing cells. (D) Incubation of SKOV ip1 human ovarian cancer cells with a C3a-receptor agonist (C3aR AG) for 2 days decreased E-cadherin mRNA level by 43% ± 0.1, and incubation with a C3a-receptor antagonist (C3aR AT) increased E-cadherin expression by 62% ± 0.2 (n=3, p<0.0001). Scrambled peptide served as the control. (E) Inhibition of KLF5 abrogated the C3a-receptor agonist-induced E-cadherin suppression (n=3, p<0.0001 and n.s= not significant). Scrambled peptide served as the control. (F) The schematic representation of the role of C3 in a pro-tumor autocrine loop in ovarian cancer. Cleavage of C3 secreted by ovarian cancer cells into tumor microenvironment generates C3a that binds to C3a-receptor on cancer cells. Activation of C3a-receptor increases KLF5, and KLF5 reduces E-cadherin expression.