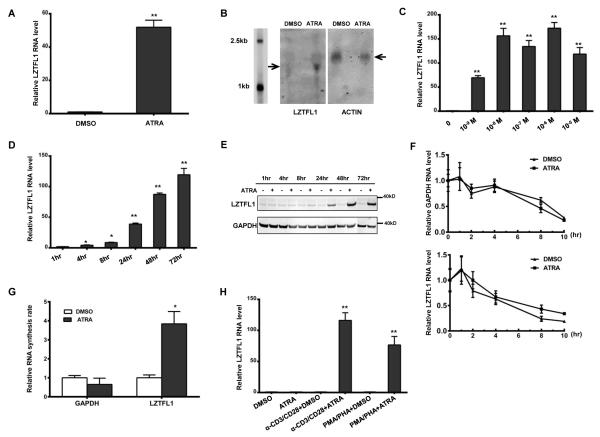
FIGURE 1.
ATRA induces LZTFL1 expression in human primary CD4+ T cells. (A) Human CD4+ T cells isolated from healthy donors were primed with anti-CD3 and anti-CD28 Abs (α-CD3/CD28) and treated with DMSO or 1 μM of ATRA for 3 d. RNAs were extracted from these cells, and LZTFL1 RNA was quantitated by reverse transcription, followed by real-time PCR. LZTFL1 RNA expression was normalized to GAPDH RNA expression, and the relative RNA fold changes compared to those from DMSO treatment were plotted (mean ± SD). (B) LZTFL1 and actin mRNAs (indicated by arrows) were detected by Northern blotting. (C–D) LZTFL1 RNA expression levels in response to different concentrations of ATRA for 72 h (C), and at 1μM ATRA for various lengths of time (D), were analyzed. (E) LZTFL1 and GAPDH proteins were detected by Western blotting. (F) CD4+ T cells treated with DMSO or ATRA for 24 h were incubated with or without actinomycin D (5 μg/ml) for 10 h and the levels of LZTFL1 and GAPDH RNAs were analyzed by real-time PCR. (G) Nuclear run-on assay was used to analyze the effect of ATRA on LZTFL1 transcription initiation. CD4+ T cells were treated with DMSO or ATRA for 24 h. Nuclei were isolated, and biotinylated transcripts were synthesized in vitro, captured, and analyzed by real-time PCR. (H) The effect of different T cell activators on LZTFL1 RNA expression was analyzed. Results are representative of three experiments with cells from three different donors. *, p < 0.05; **, p < 0.01.