Abstract
Background:
Severe and fatal complications of toxoplasmosis urge development of effective vaccines against the disease. The current study was performed to evaluate cocktail DNA vaccine containing plasmids encoding GRA5, SAG1, and ROP2 genes of Toxoplasma gondii in BALB/c mice in Tarbiat Modares University in 2012.
Methods:
The plasmids containing complete GRA5, SAG1, and ROP2 genes were mass extracted and then the recombinant plasmids were administered via intramuscular injections according to immunized mice three times with three-week intervals. Then splenocytes were cultured, and proliferation as well as cytokine assays were carried out. The other mice in each group were inoculated by the parasite and mortality of the mice was evaluated on a daily basis.
Results:
The results of cytokine assay for INF-γ were higher in the mice that received the cocktail DNA containing recombinant plasmids. Evaluation of proliferation of splenocytes using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay indicated induction of cellular response. Measurement of total IgG and the isotypes of IgG1 and IgG2a showed that the cocktail DNA stimulated IgG and IgG2a production in comparison with the control groups (P<0.05). Furthermore, the survival rate of mice in the groups that received the cocktail DNA was significantly higher than that in the control groups (P<0.05).
Conclusion:
Administration of the cocktail DNA vaccine led to production of higher levels of IFN-γ, confirmed by secretion of IgG2a, and the immune response was shifted toward Th1. Thus, the cocktail DNA containing the recombinant plasmids can be an appropriate candidate for immunization against toxoplasmosis.
Keywords: Toxoplasma gondii, Cocktail DNA Vaccine, pcGRA5, pcSAG1, pcROP2
Introduction
Contamination with Toxoplasma gondii is one of the commonest parasitic infections in human and other vertebrates (1). There is an increasing tendency towards not using antiparasitic drugs. The high-tech DNA vaccines have proposed a good perspective for development of cocktail vaccines (2–3).
In recent years, considerable achievements have been made on identification of an appropriate vaccine. Most of these attempts have focused on surface antigens. Among the antigens, SAG1 is one of the major candidates for vaccine development (2). Secretory antigens of Toxoplasma have an important role in stimulation of the protective immune system. These antigens are expressed by cystic bradyzoites as well as tachyzoites (4). The major components of secretory antigens are GRA molecules known as vaccine candidates (5). ROP2 antigen, expressed by tachyzoites, bradyzoites, and sporozoites, are considered as candidates for development of vaccines against T. gondii (6). Rhoptry proteins are associated with various cellular components and have different functions. ROP2 (54 kDa) is one the main rhoptry proteins in the T. gondii (7).
SAG1 is the main surface protein of the tachyzoites and comprises 3–5% of the total protein volume of the tachyzoites. SAG1 is a tachyzoite stage-specific protein, which is present only in tachyzoites and cannot be found in sporozoites and bradyzoites. SAG1 shows the highest immunogenicity in T. gondii tachyzoites thus, has been employed in development of cocktail DNA vaccines against toxoplasmosis (8–12).
Another group of secretory antigens is GRA, among which GRA5 is one of the main antigens of Toxoplasma secreted in different life stages of the parasite. If the whole antigen is used, it can be employed in development of effective test for detection of antibodies against toxoplasmosis (13–14).
The aim of this study was to evaluate the expression of plasmids encoding complete GRA5, SAG1, and ROP2 gens of T. gondii in eukaryotic CHO cells and immunogenicity of cocktail DNA vaccine containing these plasmids in BALB/c mice.
Materials and Methods
This research was carried out in Department of Parasitology, Faculty of Medicine, Tarbiat Modares University, Tehran, Iran in 2012 for evaluating the Cocktail DNA vaccine containing plasmids encoding complete GRA5, SAG1, and ROP2 antigens of T. gondii in BALB/C mice.
Preparation of recombinant plasmid
The construction and expression of pcDNA3 plasmids (Invitrogen, USA) contain completes genes of ROP2 gene (1,686 bp), SAG1 (960 bp) and GRA5 (363 bp) were formerly prepared and expressed in CHO cells (15–17). The primers used for each gene were as follows:
ROP2: The primers of T. gondii ROP2 were designed according to the gene sequence obtained from GenBank, with the accession No. Z36906. The PCR product size was 1686 bp. Forward primer, 32nt 5′ ATT AAG CTT ATG GAA AAC TGT GCG TCG GTC AG 3′ Hind III Reverse primer 29 nt; 5′ ATT GAA TTC TCA TGC CGG TTG TCC ATC AG 3′ EcoR I
-
SAG1: The sequence of SAG1 gene of T.gondii RH strain (complete code: 960 bp) was obtained from GenBank, with the accession No. AY217784, and the following primers were designed.
Forward primer, 27nt 5′-ATT AAG CTT ATG TTT CCG AAG GCA GTG-3′ Hind III Reverse primer, 26nt 5′-ATT GAA TTC TCA CGC GAC ACA AGC TG-3′ EcoR I
-
GRA5: The sequence of GRA5 gene of T. gondii RH strain (complete code: 363 bp) was obtained from Gen Bank, with the accession No. EU918733. The size of PCR product was 363 bp.
Forward primer 25nt, 5′- AAG CTT ATG GCG TCT GTA AAA CGC G - 3Hind III Reverse primer 27nt, 5′- GAA TTC TTA CTC TTC CTC GGC AAC TTC - 3′EcoR I. The recombinant plasmids were transformed into E. coli, strain TG1. Following mass replication of the bacterium were extracted from the bacteria using a plasmid extraction kit (Qiagen, Germany).
Animals
Female BALB/c mice at the age of 6–8 wk and bodyweight of 20±1 g were purchased from the Razi Vaccine and Serum Research Institute. The animals were kept under standard conditions of light, food, and water. The study was approved by the Ethics Committee of Tarbiat Modares University, with number 52/75793 in 23-December-2010, Tehran, Iran.
Parasite
Tachyzoites of T. gondii, strain RH, obtained from the Department of Parasitology, Tarbiat Modares University. The antigen required for the ELISA was prepared from the parasite. Moreover, the parasites were used for stimulation of peripheral blood mononuclear cells to be used in MTT and cytokine assays. The antigens obtained were measured using a 0.2 μm sterile filter (Nagle Company, USA), and the protein concentration was determined according to the Bradford method.
Immunization
Sixty female BALB/c mice at the age of 6–8 wk were divided into six groups, each including ten mice. Two groups were considered as control; one control group received 100 μl BPS and the other control group received 75 μg pcDNA3 at the volume of 100μl. Of the remaining groups, groups 3–5 respectively received injections of 50 μg of plasmids (25 μg pcSAG1 + 25 μg pcROP2, 25 μg pcSAG1 + 25 μg pcGRA5 and 25 μg pcROP2 +25 μg pcGRA5), accompanied by 25 μg pcDNA3 at the volume of 100 μl. The last cocktail group received 75 μg of a combination of the three plasmids (25 μg pcSAG1 + 25 μg pcGRA5+ 25 μg pcROP2) at equal ratio at the volume of 100 μl. The injections were performed in bilateral biceps, and in each side, the maximum injection volume was 50 μl. The immunization was performed in three steps with the interval of three weeks.
Serological tests
To perform the serological tests, serum samples were obtained from the mice at days 42 and 63 post-immunization and were kept at −20 °C until measurements. Then, IgG, IgG1, and IgG2a were measured. The IgG subtypes were evaluated using the mouse monoclonal antibody isotyping reagents (Sigma, USA). To determine the total IgG level using ELISA, 20 μg/ml Toxoplasma lysate antigens (TLA) was coated on the ELISA plate overnight. Then, after blocking and washing, the mouse sera was added to the wells at 1:10 concentration, and after washing again, anti-mouse antibody conjugated with HRP was added to the wells. As the incubation period was over, the plates were washed and TMB substrate was added. Twenty min later, reaction stopper solution, sulfuric acid 2M, was added, and the absorbance OD was read at the wavelength of 450 nm using an ELISA reader (Bio-Rad, USA). Determination of IgG1 and IgG2a were performed according to the manual of the kit (Mouse Monoclonal Antibody Isotyping Reagents, Sigma) using the ELISA method.
Evaluation of cellular immunity
Cellular immunity was evaluated by determination of lymphocyte proliferation and differentiation using the MTT and cytokine assay.
The MTT was used for evaluation of lymphocyte proliferation and differentiation. After performing the culture of peripheral blood mononuclear cells, cytokine assay was carried out for determination of IFN-γ and IL-4 levels. To this end, seven weeks after the last immunization, five mice from each group were killed and the lymphocytes were extracted from the spleen. The lymphocytes were cultured in the medium containing 20% FCS (Gibco) and RPMI 1640 (Gibco). In each well, 3×105 cells were incubated for 72 h at 37 °C in the presence of 40 μg/ml of TLA and 5% CO2 in triplicate. Then, the contents of the wells were centrifuged and until cytokine assay, the supernatant was frozen at −80 °C. Cytokine assay was performed using a kit (R&D Systems, Minneapolis, MN, USA).
To carry out the MTT assay, 20 μl of tetrazolium (Roche, Germany) at the concentration of 5 mg/ml was added to each well, and the samples were incubated at 37 °C for 4 h. Then, the plates were centrifuged at 1000 g for 10 min, the supernatant was removed, and 100 μl DMSO was added to each well. The OD values of the plates were determined at the wavelength of 540 nm.
Survival assay
To evaluate the survival rate of the mice, seven weeks after the last immunization, five mice in each group received an injection of 104 live tachyzoites of T. gondii, strain RH, and the mortality of the mice were evaluated on a daily basis.
Statistical analysis
For statistical analyses between different groups, at first the data were checked for normality distribution by Colmogorov-Smirnov test. The analyses were performed by one way ANOVA and Mann-Whitney tests. The statistically significant results are the P-value less than 0.05.
Results
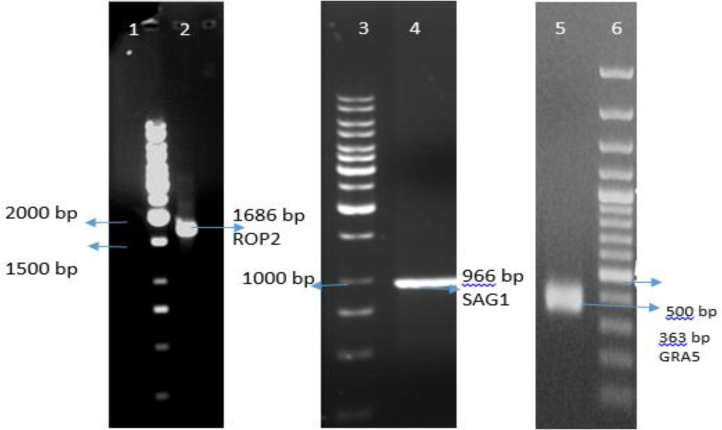
The recombinant plasmid extracted from bacteria and confirmed with PCR (Fig. 1).
Fig. 1: ROP2, SAG1 and GRA5 PCR amplification and gel electrophoresis. Lanes 1, 3 and 6 1 Kbp DNA ladder. Lane 2, PCR product of ROP2 (1686 bp); Lane 4, PCR product of SAG1 (966 bp); Lane 6, PCR product of GRA5 (363 bp).
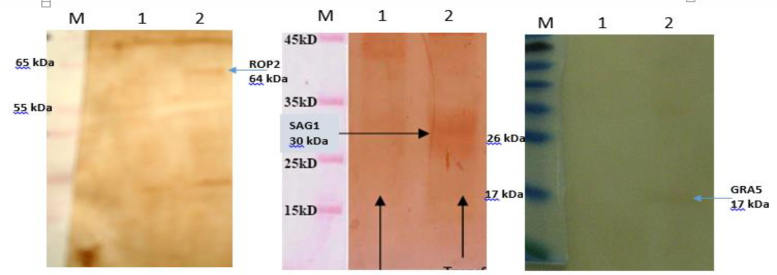
Expression of pc-ROP2, pc-SAG1 and pc-GRA5 recombinant plasmids in CHO cells after 48 h of transfection in comparison with empty pc-DNA were confirmed by SDS-PAGE and Western blot (Fig. 2).
Fig. 2: Western blot of expressed genes in CHO cells. M, protein marker; Lanes 1, transfected cells containing pc-DNA 3 (negative control); Lines 2, Lane 6, transfected cells containing pc-ROP2, pc-SAG1 and pc-gra5.
The lowest mean OD value for total IgG in the first and second blood samplings was related to the PBS group, while the highest values were obtained for the pcGRA5+ pcROP2 group (Table 1).
Table 1: Comparison of mean OD values for total IgG determined using ELISA test in serum samples of mice for two blood samplings.
| Number of group | Immunization regimen | Total IgG on day 42 | Total IgG on day 63 | ||
|---|---|---|---|---|---|
| Mean±SD | Sig ( P <0.05 ) | Mean±SD | Sig ( P <0.05 ) | ||
| 1 | pcSAG1+ pcROP2 | 0.263±0.02 | (5, 6) * | 0.31 7±0.02 | (5, 6) * |
| 2 | pcGRA5 + pcSAG1 | 0.276±0.01 | (5, 6) * | 0.310 ±0.01 | (5, 6) * |
| 3 | pcGRA5 + pcROP2 | 0.269±0.01 | (5, 6) * | 0.320±0.02 | (5, 6) * |
| 4 | pcGRA5+pcSAG1+ pcROP2 | 0.261±0.01 | (5, 6) * | 0.275±0.02 | (5, 6) * |
| 5 | PBS | 0.193±0.02 | (1، 2، 3, 4) * | 0.171±0.01 | (1, 2, 3, 4) * |
| 6 | pcDNA3 | 0.166±0.008 | (1, 2, 3, 4) * | 0.196±0.02 | (1, 2, 3, 4) * |
Significantly different from the groups considered in the parentheses according to the ANOVA test (P<0.05)
Furthermore, the cut-off value determined according to measurement of total IgG in serum samples of seronegative mice was (Meam + 3× SD) 0.199 (Table 1).
In the first and second blood samplings, the lowest mean OD values related to pcGRA5+pcSAG1 and pcGRA5+pcROP2 groups, respectively (Table 2).
Table 2: Comparison of mean OD values of IgG1 determined by ELISA in the mice in two blood samplings.
| Number of group | Immunization regimen | IgG1 on day 42 | IgG1 on day 63 | ||
|---|---|---|---|---|---|
| Mean±SD | Sig ( P <0.05 ) | Mean±SD | Sig ( P <0.05 ) | ||
| 1 | pcSAG1+ pcROP2 | 0.181±0.015 | --- | 0.157±0.013 | --- |
| 2 | pcGRA5+ pcSAG1 | 0.163±0.006 | ---- | 0.158±0.005 | ---- |
| 3 | pcGRA5+ pcROP2 | 0.204±0.01 | --- | 0.156±0.02 | --- |
| 4 | pcGRA5+pcSAG1+pcROP2 | 0.173±0.007 | --- | 0.173±0.007 | --- |
| 5 | PBS | 0.168±0.003 | ---- | 0.168±0.001 | ---- |
| 6 | pcDNA3 | 0.198±0.01 | ---- | 0.187±0.01 | ---- |
The highest values in the first and second blood samplings were related to pcGRA5+pcROP2 and pcDNA3 groups, respectively. However, the differences were not statistically significant (P>0.05). Moreover, the cut-off value determined according the IgG1 measurement in serum sample of seronegative mice was 0.168+3×0.03 =0.177 (Table 2).
According to Table 3, the lowest mean OD values in the first and second blood samplings were obtained for the pcDNA3 and PBS groups, respectively.
Table 3: Comparison of mean OD values for IgG2a determined using ELISA test in serum samples of mice for two blood samplings.
| Number of group | Immunization regimen | IgG2a on day 42 | IgG2a on day 63 | ||
|---|---|---|---|---|---|
| Mean±SD | Sig ( P <0.05 ) | Mean±SD | Sig. ( P <0.05 ) | ||
| 1 | pcSAG1+ pcROP2 | 0.252±0.01 | (5, 6) * | 0.240±0.03 | (4, 5, 6) * |
| 2 | pcGRA5 + pcSAG1 | 0.258±0.01 | (5, 6،) * | 0.282±0.01 | (4, 5, 6) * |
| 3 | pcGRA5 + pcROP2 | 0.244±0.01 | (5, 6) * | 0.244±0.02 | (4, 5, 6) * |
| 4 | pcGRA5+pcSAG1+pcROP2 | 0.266±0.02 | (5, 6) * | 0.317±0.05 | (1, 2, 3, 5, 6) * |
| 5 | PBS | 0.149±0.002 | (1, 2, 3, 4) * | 0.148±0.006 | (1, 2, 3, 4) * |
| 6 | pcDNA3 | 0.144±0.002 | (1, 2, 3, 4) * | 0.149±0.009 | (1, 2, 3, 4) * |
Significantly different from the groups considered in the parentheses according to ANOVA test (P<0.05)
This is while the highest values in the first and second samplings related to pcGRA5+pcSAG1+pcROP2 group. Furthermore, the cut-off value obtained according to IgG2a measurement in serum sample of seronegative mice was 0.155.
The lowest and the highest mean IFN-γ levels 72 hours after lymphocyte culture was related to the PBS and pcGRA5+pcSAG1+ pcROP2 groups, respectively (Table 4).
Table 4: Comparison of mean±SD IFN-γ and IL-4 levels determined by ELISA in samples of mice studied 72 hours after lymphocyte culture.
| Number of group | Immunization regimen | IFN-γ | IL-4 | ||
|---|---|---|---|---|---|
| Mean±SD | Sig (P<0.05) | Mean±SD | Sig (P<0.05) | ||
| 1 | pcSAG1+ pcROP2 | 902±155.8 | (4, 5, 6) * | 52.0±17 | (5, 6) * |
| 2 | pcGRA5 + pcSAG1 | 1062±349 | (5, 6) * | 38.4±8 | (4, 5, 6) * |
| 3 | pcGRA5 + pcROP2 | 892±196 | (4, 5, 6) * | 68±28.9 | (5, 6) * |
| 4 | pcGRA5+pcSAG1+pcROP2 | 1278±136 | (1, 3 ,5, 6) * | 120±48 | (2, 5, 6) * |
| 5 | PBS | 167±6.6 | (1, 2, 3, 4) * | 11±0.5 | (1, 2, 3, 4) * |
| 6 | pcDNA3 | 180±4.6 | (1, 2, 3, 4) * | 13±0.3 | (1, 2, 3, 4) * |
Significantly different from the groups considered in the parentheses according to the Mann-Whitney test results (P<0.05)
The lowest IL-4 levels in mice 72 h after lymphocyte culture were related to the PBS group, while the highest level was related to the pcGRA5+pcSAG1+pcROP2 group, respectively (Table 4).
Considering stimulation in the lymphocyte culture by the Toxoplasma antigen, the results obtained in the MTT assay for the experiment and control groups according to the stimulation index (SI), after the antigen stimulation in the experiment and control groups, the highest and lowest SI values were obtained as 3.29 and 1.09 for the pcGRA5+pcSAG1+pcROP2 and pcDNA3 groups, respectively (Table 5).
Table 5: Stimulation index (SI) in the MTT assay, after the lymphocyte culture stimulated with Toxoplasma gondi antigen in the experiment and control groups.
| Groups | SI |
|---|---|
| pcSAG1+ pcROP2 | 1.35 |
| pcSAG1+pcGRA5 | 1.33 |
| pcROP2+pcGRA5 | 1.36 |
| pcGRA5+pcSAG1+pcROP2 | 3.29 |
| PBS | 1.00 |
| pcDNA3 | 1.09 |
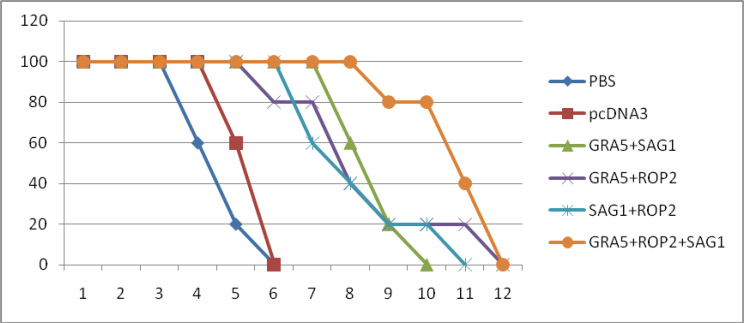
Regarding the results obtained for mortality of the mice, mortality in the two control groups; PBS and pcDNA3, started from days 4 and 5 after the challenge and all mice died until day 6.
Mortality in the pcGRA5+pcROP2 group started from day 6 after the challenge and all mice died until day 12. In the pcSAG1+pcROP2group, the mortality of mice began from day 7 and all mice died until day 11. Considering the pcGRA5+pcSAG1-+pcROP2 group, the mortality began from day 9 and all mice died until day 12. The survival rates of the mice in the days after parasitic challenge are illustrated in Fig. 3.
Fig. 3: Survival rates of immunized and control BALB/c mice after lethal challenge with 1×104 tachyzoite forms of T. gondii RH strain 7 weeks after the last immunization. Each group has five mice.
Discussion
Immunization with SAG1 and ROP2 proteins can lead to a widespread immune response, which is able to reduce the mortality rate of animals acutely contaminated by T. gondii and decrease the load of brain cyst in the infected animals (12) and the results of this study confirm the previous studies. However, the efficacy should be improved. Thus, we attempted to develop a novel vaccine by focusing on the genes encoding the major surface antigen (SAG1) (11, 17), as well as ROP2 (12, 15), and GRA5 (16).
Igarashi et al. have investigated the antigenic characteristics and production of recombinant GRA5, GRA7, and ROP2 proteins in Toxoplasma and reported the highest IFN-γ level in ROP2 plasmid. Accordingly, there was a possibility to employ these proteins in serological diagnosis or vaccination against the disease (14).
Dense granule antigens of T. gondii have been introduced as appropriate candidates for antiparasitic vaccines. Immunization with plasmids containing the afore-mentioned antigens, especially GRA5, leads to immune stimulation and antibody production (14, 18).
Gasior et al., for the first time, used complete recombinant Toxoplasma GRA5 antigen for serological diagnosis of human toxoplasmosis. Their study demonstrated that complete toxoplasma rGRA5 was appropriate to be used as a component of cocktail antigen for identification of IgG antibody against toxoplasmosis (18).
Beyond its diagnostic value, GRA5 antigen has a protective role against the disease. Igarashi et al. investigated the mechanism of protection against brain cyst formation in BALB/c mice using the recombinant inhalant vaccine of rROP2, rGRA5, and rGRA7. They reported that immunization with the vaccine mentioned together with the cholera toxin resulted in relative protection in comparison with the control group and prevented development of Toxoplasma tissue cyst after oral drug administration (19).
Zenner et al. examined the subcutaneous vaccine of GRA2 and GRA5 antigens accompanied by FIA adjuvants for Fischer rats. The treatment was effective in preventing the disease spread from mother to baby. However, effectiveness of the approach for other animal models requires further investigations (20). In another study, GRA5, GRA7, and ROP2 genes from T. gondii, strain RH, were cloned and used for production of recombinant (14).
Considering the results obtained for survival of mice after being challenged by the RH strain showed that the lowest mean survival time in the PBS and pcDNA3 control groups were 126 and 138 h, respectively. This is while the mice that received recombinant plasmids had longer survival; such that the longest survival was observed in GRA5+SAG1+ROP2 and GRA5+SAG1 groups; 234 and 207 hours, respectively.
Administration of cocktail of T. gondii recombinant plasmids led to statistically significant enhancement of the survival rate of immunized mice.
In the first sampling, the total IgG titer in the groups that received recombinant plasmid did not have statistically significant difference with that of the control groups. This is while in the second sampling, an increase was observed in the antibody level in the immunized groups, which was significantly different from the control groups (P<0.05).
Conclusion
Simultaneous administration of two or three genes (cocktail vaccine) would enhance the expression of these genes. This, in turn would increase stimulation of the immune cells by the antigens and influences the humoral response and titer of the total IgG. Moreover, the results obtained from the ANOVA and Mann-Whitney tests indicated that with regard to the total IgG titer, the control groups were significantly different from all the groups immunized by recombinant plasmids.
Considering the results obtained on a daily basis for survival rate of the mice after challenge with the RH strain, it was observed that mortality in the control groups began from day 4, and all mice dieduntil day 6 after the challenge. This is while in the GRA5+SAG1+ROP2 group, the mortality started from day 9 and all mice died until day 12.
According to the results obtained, although after challenge with live tachyzoite of T. gondii, strain RH, complete protection was not achieved in the immunized mice.
Acknowledgments
This study was supported financially by Iran National Science Foundation. The authors would like to thank the research department of Tarbiat Modares University, also Prof. Z.M. Hassan and Miss Ghasemi for their helpful aims. The authors declare that there is no conflict of interests.
References
- 1. Araujo FG. Immunization against Toxoplasma gondii. Parasitol Today. 1994; 10: 358– 360. [DOI] [PubMed] [Google Scholar]
- 2. Couvreur G, Sadak A, Fortier B, Dubremetz JF. Surface antigens of Toxoplasma gondii. Parasitology. 1988; 97: 1– 10. [DOI] [PubMed] [Google Scholar]
- 3. Capron A, Dessaint JP. Vaccination against parasitic diseases, some alternative concepts for the definition of protective antigens. Ann Inst Pasteur Immunol. 1988; 139: 109– 117. [DOI] [PubMed] [Google Scholar]
- 4. Cesbron MF, Capran A. Excreted-secreted antigens of Toxoplasma gondii: their origin and role in the host parasite interaction. Res Immunol. 1993; 144: 41– 47. [DOI] [PubMed] [Google Scholar]
- 5. Cesbron-Delauw MF. Dense granule organelles of Toxoplasma gondii: their role in the host parasite relationship. Parasitol Today. 1994; 10 (8): 293– 296. [DOI] [PubMed] [Google Scholar]
- 6. Saavedra R, Becerril MA, Dubeaux C, Lippens R, Vos MD, Herion P, Bollen A. Epitopes recognized by human T lymphocytes in the RoP2 protein antigen of Toxoplasma gondii. Infect Immun. 1996; 64: 3858– 3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadak A, Taghy Z, Fortier B, Dubremetz JF. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1998; 29: 203– 211. [DOI] [PubMed] [Google Scholar]
- 8. Velge-Roussel F, Marcelo P, Lepage AC, Buzoni-Gatel D, Bout D. Intranasal immunization with Toxoplasma gondii SAG1 induces protective cells into NALT and GALT compartments. Infec Immun. 2000; 68 (2): 969– 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nielsen HV, Lauemoller SL, Christiansen L, Buus S, Fomsgaard A, Petersen E. Complete protection against lethal Toxoplasma gondii infection in miceimmunized with a plasmid encoding the SAG1 gene. Infect Immun. 1999; 67 (12): 6358– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu KY, Zhang DB, Wei QK, Li J, Li GP, Yu JZ. Biological role of surface Toxoplasma gondii antigen in development of vaccine. World J Gastroenterol. 2006; 2363: 8– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solhjoo K, Ghaffari far F, Dalimi A, Sharifi Z. Enhancement of antibody immune response to a Toxoplasma gondii SAG1-encoded DNA vaccine by formulation with aluminium phosphate. J Med Sci. 2007; 7 (3): 361– 367. [Google Scholar]
- 12. Hoseinian Khosroshahi K, Ghaffarifar F, D'Souza S, Sharifi Z, Dalimi A. Evaluation of the immune response induced by DNA vaccine cocktail expressing complete SAG1 and ROP2 genes against Toxoplasma gondii. Vaccine. 2011; 29: 778– 783. [DOI] [PubMed] [Google Scholar]
- 13. Holec-Gasior L, Kur J. Toxoplasma gondii: Recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp Parasitol. 2010; 124 (3): 272– 8. [DOI] [PubMed] [Google Scholar]
- 14. Igarashi M, Kano F, Tamekuni K, Kawasaki PM, Navarro IT, Vidotto O, et al. Toxoplasma gondii: cloning, sequencing, expression, and antigenic characterization of ROP2, GRA5 and GRA7. Genet Mol Res. 2008; 7 (2): 305– 13. [DOI] [PubMed] [Google Scholar]
- 15. Hosseinian Khosroshahi K, Ghaffarifar F, Sharifi Z, Dalimi A. Expression of complete rhoptry protein 2 (ROP2) gene of Toxoplasma gondii in eukaryotic cell. Afr J Biotechnol. 2008; 7 (24): 4432– 6. [Google Scholar]
- 16. Naserifar R, Ghaffarifar F, Dalimi A, Sharifi Z. Cloning of Toxoplasma gondii Granular Antigen 5(GRA5) in Expression Eukaryotic Plasmid pcDNA3 and its Expression on CHO Cell.2011. J Ilam Univ Med Sci. 19(3: 1– 12. [Google Scholar]
- 17. Solhjoo K, Ghaffari far F, Dalimi A, Sharifi Z. Cloning and sequencing of Toxoplasma gondii major surface antigen (SAG1) gene. Arch Razi Inst. 2006; 61 (2): 73– 79. [Google Scholar]
- 18. Holec-Gasior L, Kur J. Toxoplasma gondii: Recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp Parasitol. 2010; 124 (3): 272– 8. [DOI] [PubMed] [Google Scholar]
- 19. Igarashi M, Kano F, Tamekuni K, Machado RZ, Navarro IT, Vidotto O, et al. Toxoplasma gondii: Evaluation of an intranasal vaccine using recombinant proteins against brain cyst formation in BALB/c mice. Exp Parasitol. 2008; 118 (3): 386– 92. [DOI] [PubMed] [Google Scholar]
- 20. Zenner L, Estaquier J, Darcy F, Maes P, Capron A, Cesbron-Delauw MF. Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted-secreted antigens as vaccine components. Parasite Immunol. 1999; 21 (5): 261– 72. [DOI] [PubMed] [Google Scholar]