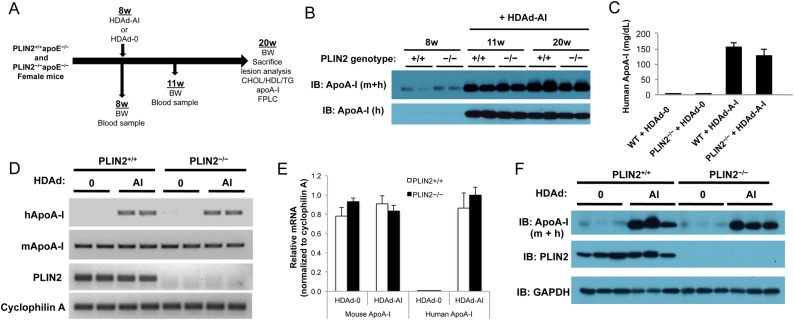
Figure 2.
Study design and HDAd-AI-mediated expression of apoA-I. (A) Schematic representation of the study design. (B) Immunoblots on plasma samples were performed with antibodies that recognize human (h) and total (mouse + human, m + h) apoA-I. PLIN2+/+/apoE–/– (+/+) and PLIN2–/–/apoE–/– (−/−) mice were bled before and after HDAd-AI injection (+HDAd-AI). While the figure displays representative examples, analysis of plasma samples from all mice included in the study yielded similar results. (C) ELISA quantification of plasma human apoA-I at the time of sacrifice (n = 11–12). (D and E) Relative RT-PCR analysis (29 cycles) (D) and qPCR analysis (n = 6) relative to cyclophilin A (E) of hepatic mouse and human apoA-I mRNA expression in PLIN2+/+/apoE–/– and PLIN2–/–/apoE–/– mice that were treated with HDAd-AI or with control empty vector (HDAd-0). (F) Immunoblots performed on liver protein lysates of PLIN2+/+/apoE–/– and PLIN2–/–/apoE–/– mice treated with HDAd-AI or with HDAd-0 (n = 3).