Abstract
AIM: To test the efficacy of gene therapy in rat liver tumor.
METHODS: A retroviral vector GCIL12EIL2PN encoding human IL-2 (hIL-2) and mouse IL-12 (mIL-12) fused gene and its packaging cell were constructed. The packaging cell lines contained of IL-2 and/or IL-12 genes were injected intrasplenically to transfect splenocyte at different time. The therapeutic effect, immune function and toxic effect were evaluated.
RESULTS: The average survival times of the 4 groups using IL genes at days 1, 3, 5 and 7 after tumor implantation were 53.3 ± 3.7, 49.3 ± 4.2, 31.0 ± 2.1 and 24.3 ± 1.4 d respectively in IL-2/IL-12 fused gene group, 25.0 ± 2.5, 23.5 ± 2.0, 18.3 ± 2.4 and 12.0 ± 1.8 d respectively in IL-2 gene treatment group, and 39.0 ± 4.8, 32.0 ± 3.9, 23.0 ± 2.5 and 19.4 ± 2.1 d respectively in IL-12 gene treatment group (P < 0.01, n = 10). In the IL-12/IL-2 fused gene treatment group, 30% of rats treated at days 1 and 3 survived more than 60 d and serum mIL-12 and hIL-2 levels were still high at day 3 after treatment. Compared with IL alone, NK cell activity was strongly stimulated by IL-2/IL-12 gene. Microscopy showed that livers were infiltrated by a number of lymphocytes.
CONCLUSION: IL-2 and/or IL-12 genes injected directly into spleen increase serum IL-2 and IL-12 levels and enhance the NK cell activity, which may inhibit the liver tumor growth. The therapy of fused gene IL-2/IL-12 is of low toxicity and relatively high NK cell activity. Our data suggest that IL-2/IL-12 fused gene may be a safe and efficient gene therapy for liver tumor. The gene therapy should be administrated as early as possible.
INTRODUCTION
Gene therapy to liver cancer is limited by both number and duration of died cancer cells being treated[1,2]. Interleukin 2 (IL-2) and interleukin 12 (IL-12) were secreted mainly by mononuclear cell and B cell[3], which play a prominent role in immune response to tumor. These cytokines are stimulated by antigens, for instance virus, bacteria and tumor cells. Others have shown that IL-2 and IL-12 were inhibited by colon cancer[4] and both can up-regulated the T-cell and NK cell to kill tumor cell after administration of exogenous ILs.
The conditions under which the gene therapies of IL-2 and IL-12 are cytotoxic to liver tumor in an animal model have not been clear yet. Understanding the role and the mechanism of IL-2 and IL-12 in the induction of anti-tumor cytotoxic factors is relevant to both the long-term expression of IL and the safety of gene drug in liver tumor gene therapy.
IL-2, a glycoprotein consisted of 133 amino acids, through promotion of growth and proliferation of T cell, can induce either LAK cell or NK cell. IL-2 also induces immunocyte to produce interferon (INF) and tumor necrosis factor (TNF). Immunoreaction mediated by IL-2 increases therapeutic efficacy of colon cancer[4,5]. Secretion of IL-2 and reaction to IL-2 are decreased in tumor patients by as yet unknown reasons. Previous report has demonstrated that metastases are related to the IL-2 level, and the tumor immune mediated by IL is decreased in favor of carcinogenesis, growth, diffusion and metastasis of tumor[6-10].
IL-12 has p35 subunit and p40 subunit localized respectively on chromosome 3 and chromosome 5. The single subunit has no biological activity, and p35 gene and p40 gene are expressed simultaneously to have activity. IL-12 activates NK cell and LAK cell, promotes generation and differentiation of T cell and induces NK cell to express a small amount of TNF-β .
Primary liver cancer is common malignant tumors in China. The surgical treatment of the cancer is a main choice, but it is not ideal therapy because of high recurrence. Gene therapy is the new way for some diseases such as diabetes and tumors. Our previous work was to treat liver cancer with IL-12 gene alone, by which tumor growth was inhibited compared with control. Now we constructed the fused gene of IL-2/IL-12. However, little is known on liver cancer treatment with combination of IL-2/IL-12 gene therapy
We hypothesized that the immunologic regulation to liver cancer following fused gene therapy of IL-2/IL-12 would be dependent upon the production and duration of the cytokines. The toxic effect of IL-2 and IL-12 would be dependent upon approach of drug delivery. In this study, using the model of implanted hepatoma of rats, we have shown that the liver tumors are reduced after IL-2 and/or IL-12 gene are injected into spleen and the cytokines are transferred into liver and blood circulation by porta vein.
MATERIALS AND METHODS
Construction of retrovirus vector of packaging mIL-12 gene and hIL-2 gene
Aprotinin, leupeptin, pepstatin, fetal calf serum and protein standard mixture were from Sigma. Plasmid pGCp35IRESp40 containing both p35 subunit and p40 subunit of mIL-12 gene was a gift from Professor Xin-Huan Liu. Plasmid pLIL2SN containing hIL -2 gene was constructed by our laboratory. Cellular strains of hepatoma CBRH3 were provided kindly by Professor Hong Xie (Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences). Monotropic packaging cell PE501, amphophilic packaging cell PA317 and cell NIH3T3 were provided by our laboratory. All other chemicals were of analytical grade and obtained from Merck or Sigma.
To amplify full length p35 and p40 genomes, PCR fragment was generated from downstream primer of mIL-12 p35 subunit and upstream primer of mIL-12 p40 subunit that templated with pGCp35IRESp -40SN. The products derived from PCR were harvested after 1% agarose gel electrophoresis. According to pLIL2PN templation, hIL-2 genome was then amplified and harvested. Fragments of mIL-12 p35 and p40 derived from PCR were templated to amplify with downstream primer of p35 and upstream primer of p40 by PCR, which contain linker sequence. The mIL-12 fused gene was harvested by electrophoresis[11,12]. The mIL-12 and hIL-2 gene fragments were connected respectively with pGEMTM -Teasy vector (Promega, USA) by T4 ligase (Boehringer Mannheim, Germany). The vector was transfected into E.coli TG1 competence cell and the positive clone was screened by PCR. Plasmid DNA was extracted from positive clone after partial exonuclease III digestion of PCR product, resulting in constructing GCILEXPN polyclonal sites after endonulease (Not I and Sal I ) (Promega, USA) that containing GCIL12EXPN of mIL-12 gene, GCXEIL2PN of hIL-2 gene and GCIL12EIL2PN of mIL-12 and hIL-2 fused gene.
Retrovirus packaging, identification, titer determination and expression
Reverse transcript virus was transfected into PE501 cell by electroporation. The clones were screened by G418 after 48 h. PA317 cell was infected by filtered supernatant 10 -14 d late. G418 was screen after 3 d. Viral supernatants were harvested after amplifying 6 monclone from every groups for 2-3 wk. Stock cells were frozen at -80 °C.
Recombined reverse transcript viral vector was identified by RT-PCR. Titers of viral supernatants were determined with NIH3T3 cell. The packaging cells with highest viral titer that contain GCIL12EXPN, GCXEIL2PN and GCIL12EIL2PN were named as PA317-GCIL12EXPN, PA317-GCXEIL12PN and PA317-GCIL12EIL2PN, respectively. Protein expression of mIL-12 and hIL-2 was determined by ELISA. ELISA kits (human IL-2 DuoSet and mouse IL-2 DuoSet) were from R&D Systems.
Implanted liver cancer in rats
Male Wister rats (200-250 g bm) were obtained from Animal Center of Chinese Academy of Sciences. Animals were maintained on a standard diet. Hepatoma CBRH3 cells were injected into abdominal cavity of rat. Rats were sacrificed and tumors were removed from abdominal cavity 7-9 d late. Tumors were cut into pieces of 0.05-0.75 cm and then were implanted into rat liver for one or more locations respectively. The tumors were grown up to 0.6-1 cm in diameter after 7-10 d of implantation.
Experimental procedure
Rats were anaesthetized with diethylether. The effects of IL-2 and/or IL-12 on liver cancer were studied in a total of 75 animals for 5 groups that contained 15 rats in every group: 1. Physiological saline control: 0.8 mL 9 g/L NaCl was injected into spleen following implanted liver cancer at day 1; 2. Blank vector control: 1 × 107 packaging cells of PA317-GCXEPN was injected into spleen of rat; 3. mIL-12 gene group: 1 × 107 packaging cell PA317-GCIL12EXPN was injected into rat spleen after implanted rat liver cancer on days 1, 3, 5 and 7 thereafter; 4. hIL-12 gene group: 1 × 107 packing cell PA317-GCxeILPN was injected into rat spleen after implanted rat liver cancer on days 1, 3, 5 and 7 respectively; 5. hIL-2 and mIL-12 fused gene group: 1 × 107 packing cell PA317-GCIL12EIL2PN was injected into rat spleen after implanted rat liver cancer on days 1, 3, 5 and 7 thereafter. In addition, when rat survived over 2 mo, rats were named long term survivors and the liver cancer tissues were implanted into liver once more in order to observe cancer growth.
CT imaging and pathology
CT imaging was observed before and after treatment. The survival time and drug toxicity were observed. To observe the tumor cell and lymphocyte infiltration, the pathologic examination was performed following 5 and 7 d of treatment. The serum IL-2 and IL-12, according to the protocol of R&D Systems, were measured on d 1 before treatment and on d 3,7, 30 and 60 thereafter.
Analysis of NK cytotoxic activity
NK target cell YAC-1 was obtained from the American Type Culture Collection (Bethesda, MD). The cytotoxicity of spleen NK cell was analyzed as follows[13]. A single -cell suspension of spleen cells was centrifuged at 400 r/min for 30 min. The lymphocyte layers were harvested. For the preparation of targeted cells, YAC-1 was labeled with [51Cr]Na2CrO4 and mixed with various numbers of spleen cell in a total volume of 200 μL of DMEM. The experimental radioactivity released (ER) in 100 μL samples of cell-free supernatants was determined. The amounts of radioactivity released in wells containing YAC-1 cells alone with and without 0.01% Triton X-100 were designated the total release (TR) and the spontaneous release (SR), respectively. The percentage of specific 51Cr release was calculated by[(ER-SR)/(TR-SR)] × 100.
Statistical analysis
All results were expressed as the mean ± SD of at least 10 individual measurements. A one-way analysis of variance (ANOVA) was first carried out to test for any differences in mean values between experimental and respective control group. If differences were established, the values were compared by two-tailed unpaired t test. The values were considered to significant difference if P < 0.05.
RESULTS
Identification of packing cell strain
The total RNA of packaging cell strains (PA317-GCIL12EXPN, PA317-GCXEIL2PN and PA317- GCIL12EIL2PN) was extracted. The RNA was amplified by RT-PCR and the product was harvested by electrophoresis. The sequence showed that PA317-GCXEIL2PN and PA317-GCIL12EIL2PN packaging cell strains contained the mIL-12 sequence, and PA317-GCXEIL2PN and PA317-GCIL12EIL2PN packaging cell strains contained the hIL-2 sequence. The target genes were inserted into viral genomes and the packaging cell strains were transfected by IL gene.
Determination of titer of virus and protein expression
The highest virus titer of packaging cell was 2 × 106 CFU/mL of PA317-GCIL12EXPN, 2.4 × 106 CFU/mL of PA317-GCXEIL2PN and 1.4 × 106CFU/mL of PA317-GCIL12EIL2PN respectively. Protein expression was measured by ELISA on these 3 packaging cell strains: 1. mIL -12 fused protein: PA317-GCIL12EXPN expressed mIL-12 fused protein 150 ng/106/48 h and PA317-GCIL12EIL2PN expressed mIL-2 fused protein 45.8 ng/106/48 h; 2. hIL-2 protein: PA317-GCEXIL2PN expressed hIL-2 protein 7.5 ng/106/48 h and PA317-GCIL12EIL2PN expressed hIL-2 protein 6.7 ng/106/48 h.
Average survival time by IL gene therapy
Table 1 shows the average survival time of rat. All rats were died within 15 d after implanting liver cancer in both physiologic saline control and blank vector control. Compared with control, the survival time of rat was prolonged significantly by treatment with IL-2 or IL-12 (P < 0.01). The rat survival time of treatment with fused gene of IL-2/IL -12 was lengthened markedly compared with IL treatment alone (P < 0.05). Moreover, the survival time of early treatment was much longer than that of later treatment with IL (P < 0.01). In addition, 6 rats treated early with fused gene of IL-2/IL-12 were lived more than 60 d, but there were 3 rats treated with IL-12 that lived more than 60 d.
Table 1.
Average survival time (day) of rat (n = 0, mean ± SD)
| Group | Injection on d 1 | Injection on d 3 | Injection on d 5 | Injection on d 7 |
| Physiologic saline control | 10.7 ± 1.5 | - | - | - |
| Blank vector control | 11.4 ± 1.3 | - | - | |
| IL-2 gene therapy | 25.0 ± 2.5b | 23.5 ± 2.0b | 18.3 ± 2.4b | 12.0 ± 1.8 |
| IL-12 gene therapy | 39.0 ± 4.8b | 32.0 ± 3.9b | 23.0 ± 2.5b | 19.4 ± 2.1a |
| IL-12-IL-2 fused gene therapy | 53.3 ± 3.7c | 49.3 ± 4.2c | 31.0 ± 2.1c | 24.3 ± 1.4c |
P < 0.05 and
P < 0.01 vs physiologic saline control and blank vector control;
P < 0.05 vs IL-2 group or IL-12 group.
When the tumor was undetectable by abdominal pathologic biopsy in the rats that lived more than 60 d, the 4 rats were implanted tumor piece again. It was interested that there were no tumor growths in abdominal cavities 7 d late.
Imaging features
The liver tumor was detected by CT on day 7 after implantion in control (Figure 1). The 6 of 10 rats administrated early with IL-2/IL-12 fused gene showed that the liver tumors were reduced after 2 mo (Figure 2).
Figure 1.
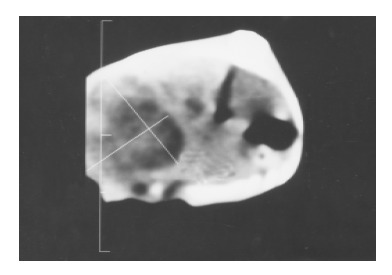
CT scan of the liver cancer 7 d after implantation.
Figure 2.
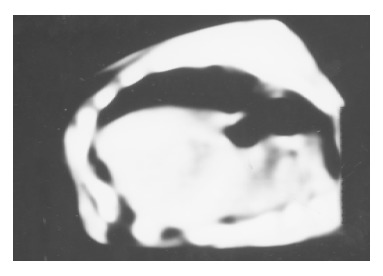
CT scan of the tumor treated with IL-2/IL-12 fused gene after 2 mo.
Pathologic features
A number of lymphocyte infiltrations in liver tumor were observed significantly after 5 d of treatment with IL-2/IL-12 fused gene and 7 d of treatment with IL-2 gene or IL-12 gene (Figure 3).
Figure 3.
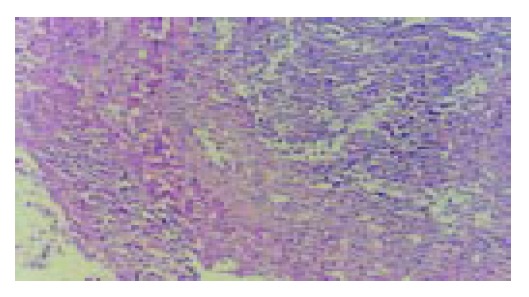
Pathological changes of implanted liver cancer (HE staining, original magnification: × 200). There are numerous lymphocytes in tumor tissues.
Serum mIL-12 and hIL-2 levels
Compared with control, both IL-2 and IL-12 in serum were increased significantly in rats treated with IL gene on day 1 (Table 2). After the injection of IL-2/IL-12 fused gene, both IL-2 and IL-12 in serum reached the highest level on day 3, then decreased stepwise and maintained at a lower level for 2 mo (Table 3).
Table 2.
Determination of serum hIL-2 or mIL-12 3 d after administration of IL gene (ng/mL)
| Group | HIL-2 | MIL-12 |
| Physiologic saline control | < 0.8 | < 0.8 |
| Blank vector control | < 0.8 | < 0.8 |
| IL-2 gene therapy | 19.4 ± 1.8 | < 0.8 |
| IL-12 gene therapy | < 0.8 | 22 ± 2.5 |
| IL-12-IL-2fused gene therapy | 18.5 ± 2.4 | 20.5 ± 2.5 |
Table 3.
Determination of serum hIL-2 and mIL-12 on fused gene group (ng/mL)
| Group | HIL-2 | MIL-12 |
| Control | < 0.8 | < 0.8 |
| On d 3 after therapy | 18.5 ± 2.4 | 20.5 ± 2.5 |
| On d 7 after therapy | 10.2 ± 2.5 | 11.5 ± 2.5 |
| One mo after therapy | 5.3 ± 1.2 | 6.2 ± 1.4 |
| Two mo after therapy | < 0.8 | < 0.8 |
IL increases NK cell activity
IL-2 and IL-12 have been reported to increase NK cell activity. To determine if the IL-2/IL-12 fused gene could induce NK cell activation, rats were treated as described above with IL-2 and/or IL-12. The rats were sacrificed on day 7, and the spleen lymphocytes were assayed for the ability to kill 51Cr-labeled YAC-1 target cells. There was a significant increase in NK activity after rats were injected with IL-2 or IL-12 alone (P < 0.01). Compared with IL alone, treatment with IL-2/IL-12 fused gene markedly enhanced NK cell activity (P < 0.05).
Toxicity of IL
Three rats (30%) in IL-2 group and 1 (10%) in IL-2/IL-12 group showed anorexia after administration of IL. The symptom was recovered 3-5 d late (Figure 4).
Figure 4.
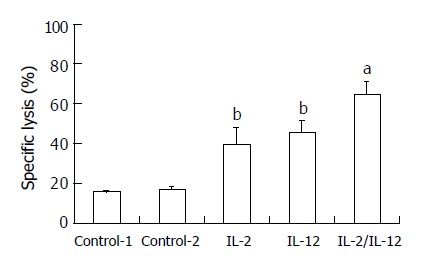
Activation of NK cell activity after administration of IL-2 and/or IL-12 (n = 5). bP < 0.01 vs control; aP < 0.05 vs IL-2 group or IL-12 group.
DISCUSSION
The establishment of DNA recombination and transfection allowed us to utilize gene therapy for killing or inhibiting the tumor cells[14]. The utility of recombinant adenoviral vectors for gene therapy is limited by the low transduction efficiency and lack of specificity for target cells[15,16]. Spread of the virus throughout the cells maximizes the percent of cells within a cell expressing the gene of interest, and should improve the antitumor response. This may be significant for gene delivery expressing cytokines, tumor antigens or enzymes. One limitation of the transduction system is the inefficiency of the combined cytokines expression system. The initial work in this study was to construct the IL- 2/IL-12 fused gene vector that was then injected into spleen, in which splenic cells, probably including liver cells, were transducted to express IL-2 and IL-12 simultaneously.
The active IL-12 is a heterogeneous dimmer, which contains p35 subunit and p40 subunit, so that both subunits express simultaneously in cells if used to treat disease. Using PCR technique based on the subunit characterization of different gene sequences, we constructed the active IL-2/IL-12 fused gene and a linker sequence was incorporated into subunits of IL-12, so that the gene could correctly express protein. In present study, the IL-2 and IL-12 levels were not significantly different in serum.
The single gene therapy for the liver cancer is not ideal because it is resulted from multi-factors[17]. Chen et al suggested recently that TRAIL and chemotherapeutic agents or anticancer cytokines combination might be a novel strategy for the treatment of liver cancer. Combination of IL-2/IL-12 results in stable antitumor effect, which induces the cytotoxic T lymphocyte and NK cell[18]. IL-2 and IL-12 have synergetic effect such as immunoregulatory[19,20]. The antitumor immunologic effect from IL-2 and IL-12 depends on available concentration of IL-2 and IL-12. IL-2 and IL-12 also cause a long term antitumor immunologic memory [21]. In present study, retrovirus vector containing IL-2/IL-12 genes was constructed, in which gene products of IL-2 and IL-12 were expressed simultaneously in the liver. We treated rat liver cancer with IL- 2 and/or IL-12 gene. The liver cancer rats treated with IL-2 or IL-12 survived longer than those in control (P < 0.01). Compared with IL alone, combination of IL-2/IL-12 gene showed a longer survival of 14-28 d in early treatment group and 5-12 d in late treatment group.
Spleen is the biggest immune organ that has a lot of immunocytes and produces antibodies and cytokines. Spleen is also the main organ of inducing immunoresponsiveness to heterologous antigen. The retrovirus packaging cell strain was injected into spleen, which expresses the high level of cytokine in order to activate immunocytes. In other hand, the retrovirus packaging cells would be transferred from spleen to liver by splenic veins and the liver cells would be infected by packaging cells resulting in enhanced anti-tumor immunity. In present study, the IL gene was injected into spleen and the blood concentration of IL was the highest on day 3. The IL concentration maintained at a level for 2 mo. Authors from Spain reported that gutless adenoviral vector encoding hIL-2 and mIL-12 was injected into animals and IL was expressed by hepatocytes. The peak concentration of IL-12 was at 10 h and it completely disappeared by 72 h. If the vectors were administrated continually, the serum IL-12 would maintain at least for 48 wk[22,23]. The rats received the splenic gene therapy survived longer than those in control. When combination of IL-2/IL-12 gene was injected into spleen, the high concentration of IL was determined from blood until day 3 and the rats survived a longer time compared with IL therapy alone.
Moreover, early gene treatment is better than late therapy. In this study, 6 rats (30%) with combined gene therapy on early stage survived a long term and the tumor nodes in liver was not detected by CT imaging and pathologic observation, in which the IL-2 and IL-12 kept a high concentration at least for 2 mo. As same as other therapy for cancer, the IL gene therapy should administrate as early as possible. In present study, the liver cancer rats treated with IL-2 or IL-12 on day 1 survived more than 8 d or 13 d respectively compared with that treated on d 7 (Table 1). The combined IL gene therapy has the similar result.
IL-2 or IL-12 produced significant toxic reaction if they were administrated enough dosage to maintain a high blood concentration. When IL-2 or IL-12 was injected into rat liver, some rats showed acute toxic reaction such as anorexia, convulsion and bleeding shock (unpublished data). In present study, when IL genes were injected into spleen, the severe acute toxic reaction was not observed in all groups.
A mechanism of IL gene treatment may be due to enhancement of NK cell activation and production of cytokines including IL[24]. Several investigators have shown that NK cells are a relative smaller cell population in peripheral lymphoid organs but are abundant in the liver. An initial response to tumor cell may involve the innate arm of the immune response resulting in killing of mutant cell strain by NK cell[25,26]. The findings in this study are novel since IL -2/IL-12 fused gene expresses IL-2 and IL-12 simultaneously that result in further stimulation of NK cells. We propose that liver tumor is inhibited because of IL production, such as IL-2 and IL-12, which is stimulated by IL gene therapy.
It is interesting that serum IL -2 and IL -12 levels do not change in IL-2/IL-12 gene therapy compared with IL alone, but NK cell activity is enhanced significantly compared with IL alone. We propose that the NK cell activity is strongly stimulated by both IL-2 and IL-12 at the same time, which was caused by IL-2/IL-12 fused gene expression[27-30].
In summary, treatment with IL-2 and/or IL-12 gene increases serum IL-2 and IL-12 levels and enhances the NK cell activity, which may inhibit the liver tumor growth. The fused gene therapy of IL-2/IL-12 is of low toxicity and relatively high NK cell activity. We suggest that IL-2/IL-12 fused gene therapy may be a safe and efficient method for the treatment of liver cancer. For IL gene therapy, early intervention is better than late one.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30271476 and No. 39970838 and the Shanghai Science and technology Key Problem Foundation, No. 034119837
Edited by Zhang JZ and Chen WW Proofread by Xu FM
References
- 1.Yang Y, Wilson JM. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995;155:2564–2570. [PubMed] [Google Scholar]
- 2.Shi M, Wang FS, Wu ZZ. Synergetic anticancer effect of combined quercetin and recombinant adenoviral vector expressing human wild-type p53, GM-CSF and B7-1 genes on hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9:73–78. doi: 10.3748/wjg.v9.i1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 4.Nakamori M, Iwahashi M, Nakamura M, Ueda K, Zhang X, Yamaue H. Intensification of antitumor effect by T helper 1-dominant adoptive immunogene therapy for advanced orthotopic colon cancer. Clin Cancer Res. 2003;9:2357–2365. [PubMed] [Google Scholar]
- 5.Chi CH, Wang YS, Lai YS, Chi KH. Anti-tumor effect of in vivo IL-2 and GM-CSF electrogene therapy in murine hepatoma model. Anticancer Res. 2003;23:315–321. [PubMed] [Google Scholar]
- 6.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 7.Milanovich MR, Snyderman CH, Wagner R, Johnson JT. Prognostic significance of prostaglandin E2 production by mononuclear cells and tumor cells in squamous cell carcinomas of the head and neck. Laryngoscope. 1995;105:61–65. doi: 10.1288/00005537-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Arvind P, Papavassiliou ED, Tsioulias GJ, Qiao L, Lovelace CI, Duceman B, Rigas B. Prostaglandin E2 down-regulates the expression of HLA-DR antigen in human colon adenocarcinoma cell lines. Biochemistry. 1995;34:5604–5609. doi: 10.1021/bi00016a035. [DOI] [PubMed] [Google Scholar]
- 9.Pavlidis N, Nicolaides C, Bairaktari E, Kalef-Ezra J, Athanassiadis A, Seferiadis C, Fountzilas G. Soluble interleukin-2 receptors in patients with advanced colorectal carcinoma. Int J Biol Markers. 1996;11:6–11. doi: 10.1177/172460089601100102. [DOI] [PubMed] [Google Scholar]
- 10.Satomi A, Murakami S, Ishida K, Mastuki M, Hashimoto T, Sonoda M. Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol. 1995;34:69–73. doi: 10.3109/02841869509093641. [DOI] [PubMed] [Google Scholar]
- 11.Merchlinsky M, Moss B. Resolution of vaccinia virus DNA concatemer junctions requires late-gene expression. J Virol. 1989;63:1595–1603. doi: 10.1128/jvi.63.4.1595-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnant MF, Noll LA, Irvine KR, Puhlmann M, Terrill RE, Alexander HR, Bartlett DL. Tumor-specific gene delivery using recombinant vaccinia virus in a rabbit model of liver metastases. J Natl Cancer Inst. 1999;91:1744–1750. doi: 10.1093/jnci/91.20.1744. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HG, Xie J, Xu L, Yang P, Xu X, Sun S, Wang Y, Curiel DT, Hsu HC, Mountz JD. Hepatic DR5 induces apoptosis and limits adenovirus gene therapy product expression in the liver. J Virol. 2002;76:5692–5700. doi: 10.1128/JVI.76.11.5692-5700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–149. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 15.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 16.Walker JR, McGeagh KG, Sundaresan P, Jorgensen TJ, Rabkin SD, Martuza RL. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther. 1999;10:2237–2243. doi: 10.1089/10430349950017211. [DOI] [PubMed] [Google Scholar]
- 17.Salazar-Mather TP, Hamilton TA, Biron CA. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, Lo KM. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51:449–460. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietrich A, Kraus K, Brinckmann U, Friedrich T, Müller A, Liebert UG, Schönfelder M. Complex cancer gene therapy in mice melanoma. Langenbecks Arch Surg. 2002;387:177–182. doi: 10.1007/s00423-002-0299-5. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Shugert E, Guo M, Bishop JS, O'Malley BW. Combination nonviral interleukin 2 and interleukin 12 gene therapy for head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2001;127:1319–1324. doi: 10.1001/archotol.127.11.1319. [DOI] [PubMed] [Google Scholar]
- 21.Wigginton JM, Wiltrout RH. IL-12/IL-2 combination cytokine therapy for solid tumours: translation from bench to bedside. Expert Opin Biol Ther. 2002;2:513–524. doi: 10.1517/14712598.2.5.513. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Hernández-Alcoceba R, Shankar V, Zabala M, Kochanek S, Sangro B, Kramer MG, Prieto J, Qian C. Prolonged and inducible transgene expression in the liver using gutless adenovirus: a potential therapy for liver cancer. Gastroenterology. 2004;126:278–289. doi: 10.1053/j.gastro.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 23.Sobota V, Bubeník J, Símová J, Jandlová T. Intratumoral IL-12 gene transfer improves the therapeutic efficacy of IL-12 but not IL-19. Folia Biol (Praha) 2000;46:191–193. [PubMed] [Google Scholar]
- 24.Tanaka M, Saijo Y, Sato G, Suzuki T, Tazawa R, Satoh K, Nukiwa T. Induction of antitumor immunity by combined immunogene therapy using IL-2 and IL-12 in low antigenic Lewis lung carcinoma. Cancer Gene Ther. 2000;7:1481–1490. doi: 10.1038/sj.cgt.7700251. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Timiryasova TM, Haghighat P, Andres ML, Kajioka EH, Dutta-Roy R, Gridley DS, Fodor I. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001;24:46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, Watkins SC, Todo S, Herberman RB, Lotze MT, et al. Local administration of IL-12-transfected dendritic cells induces antitumor immune responses to colon adenocar-cinoma in the liver in mice. J Exp Ther Oncol. 2002;2:337–349. doi: 10.1046/j.1359-4117.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 27.Sangro B, Qian C, Schmitz V, Prieto J. Gene therapy of hepatocellular carcinoma and gastrointestinal tumors. Ann N Y Acad Sci. 2002;963:6–12. doi: 10.1111/j.1749-6632.2002.tb04089.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato T. Locoregional immuno(bio)therapy for liver metastases. Semin Oncol. 2002;29:160–167. doi: 10.1053/sonc.2002.31716. [DOI] [PubMed] [Google Scholar]
- 29.Martinet O, Divino CM, Zang Y, Gan Y, Mandeli J, Thung S, Pan PY, Chen SH. T cell activation with systemic agonistic antibody versus local 4-1BB ligand gene delivery combined with interleukin-12 eradicate liver metastases of breast cancer. Gene Ther. 2002;9:786–792. doi: 10.1038/sj.gt.3301687. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Katayose Y, Unno M, Suzuki M, Kodama H, Takemura S, Asano R, Hayashi H, Yamamoto K, Matsuno S, et al. A novel adenovirus expressing human 4-1BB ligand enhances antitumor immunity. Cancer Immunol Immunother. 2003;52:97–106. doi: 10.1007/s00262-002-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


