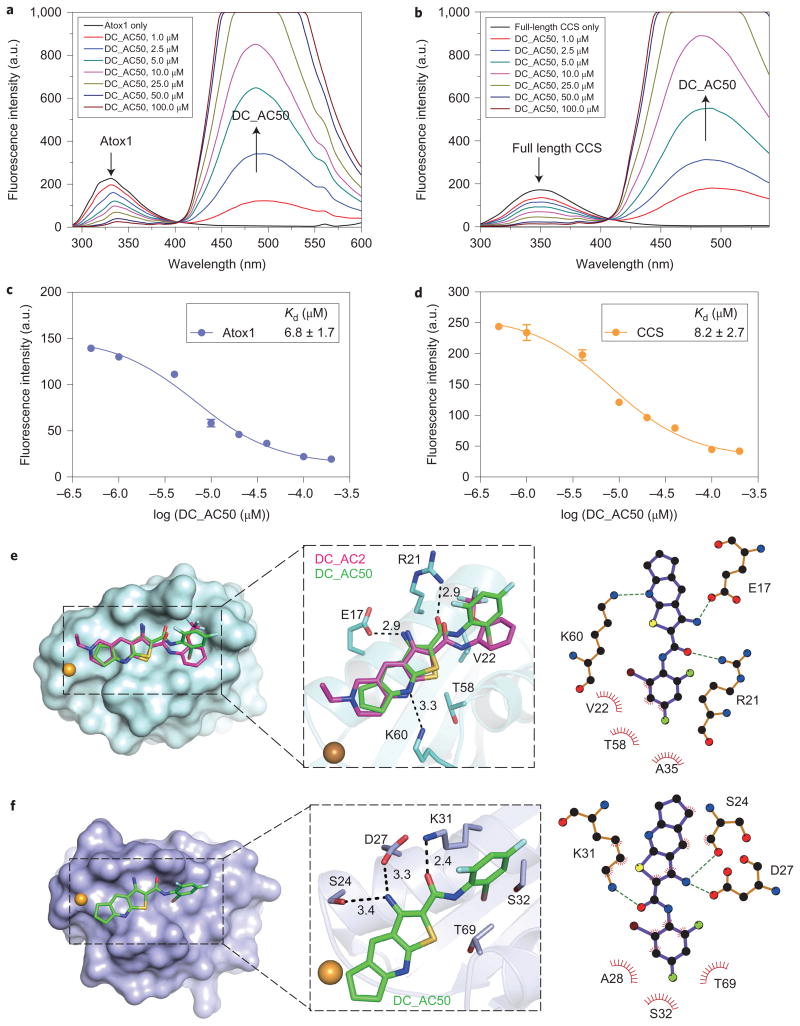
Figure 2. Docking model and binding of DC_AC50 to Atox1 and full-length CCS by FRET measurements (from Tyr/Trp to DC_AC50).
a,b, FRET between Atox1 (Tyr), full-length CCS (Trp) and DC_AC50. Atox1 or full-length CCS (1 μM) displayed the maximum fluorescence emission at 335 or 350 nm, respectively, in the absence of DC_AC50. With the addition of DC_AC50 (1-100 μM and less than 5 μl DMSO in 200 μl buffer), the peak at 335 or 350 nm, which corresponds to the emission of Tyr or Trp, respectively, was reduced, whereas the emission of DC_AC50 at 494 nm was increased. FRET changes are shown in coloured lines. c,d, Binding curves of DC_AC50 to Atox1 and CCS. The experiments were performed in 50 mM HEPES, 200 mM NaCl, 1 mM DTT (pH 7.1). e,f, Surface representations that show DC_AC50 (green) and DC_AC2 (purple) binding to Atox1 (e; PDB 1TL4) and CCS (f; PDB 2CRL). Error bars, mean ± s.e.m., n = 3 biological replicates.