Figure 1.
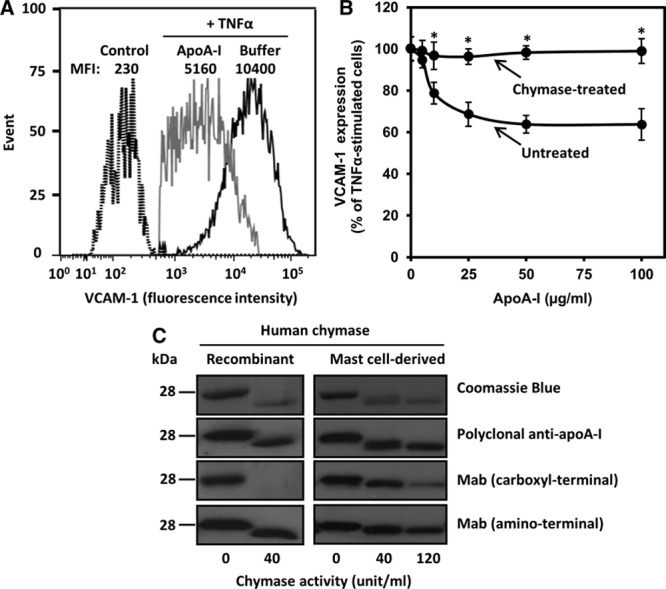
Chymase treatment abolishes the anti-inflammatory activity of apolipoprotein A-I (apoA-I) on activated human coronary artery endothelial cells (HCAECs). A, HCAECs were preincubated for 16 h in the absence (buffer) or presence of apoA-I (50 μg/mL) and then activated with tumor necrosis factor-α (TNF-α; 10 ng/mL) for 5 h. Nonactivated cells were incubated in medium alone for 21 h (control). Cell surface vascular cell adhesion molecule-1 (VCAM-1) protein was determined by flow cytometry. Data are representative of 6 independent experiments. B, ApoA-I (1 mg/mL) was treated for 6 h in the absence (untreated) or the presence (chymase-treated) of chymase (0.5 μg=40 BTEE units/mL). HCAECs were preincubated with increasing concentrations of the untreated or chymase-treated apoA-I and then activated with tumor necrosis factor-α (TNF-α), as described in A. TNF-α-induced VCAM-1 surface protein expression was analyzed by flow cytometry and expressed as percentage of its expression levels in TNF-α-activated cells preincubated in the absence of apoA-I, which was set as 100%. Data represent the means±SD from 3 to 4 independent experiments performed in duplicate. *P<0.01 denotes statistical significance between cells preincubated with untreated or chymase-treated apoA-I. C, ApoA-I was incubated with the indicated activities (BTEE units) of recombinant human chymase or chymase-containing human mast cell–conditioned medium for 6 h, after which the incubation was stopped by adding soybean trypsin inhibitor. Proteins in the incubation mixtures were resolved in 12.5% SDS-PAGE and detected by Coomassie Blue or immunoblotted with anti-human apoA-I polyclonal antibody or with anti-human apoA-I monoclonal antibodies recognizing either a C-terminal (amino acids 211–220) or an N-terminal (amino acids 2–8) region of apoA-I. MFI indicates median fluorescence intensity.
