Figure 2.
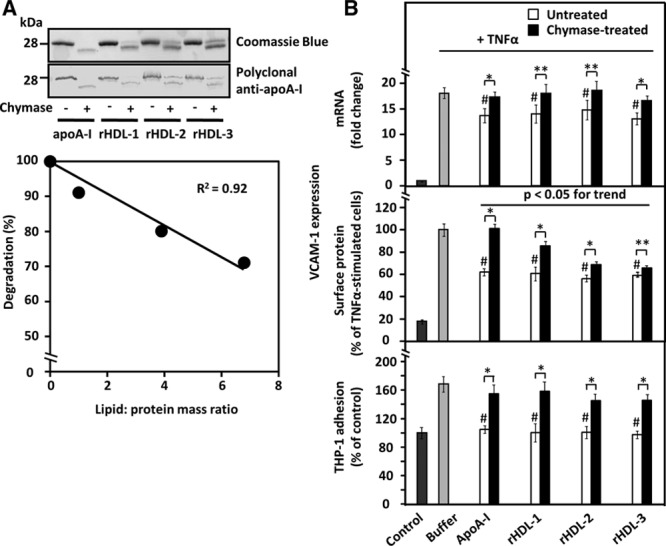
Effect of apolipoprotein A-I (apoA-I) lipidation on its sensitivity to proteolysis and on the ability of untreated and chymase-treated apoA-I to influence vascular cell adhesion molecule-1 (VCAM-1) expression in and THP-1 adhesion to tumor necrosis factor-α (TNF-α)-activated human coronary artery endothelial cells (HCAECs). A, Top, ApoA-I and discoidal apoA-I-containing reconstituted high-density lipoprotein ((A-I)rHDLs) with 3 different degrees of lipidation (see Table I in the online-only Data Supplement) were incubated in the absence or presence of recombinant chymase, and the proteins in the incubation mixtures were analyzed, as described in Figure 1. Bottom, ApoA-I degradation was expressed as a percentage of the intensity of the intact band in the proteolyzed samples relative to the corresponding untreated sample and plotted against the lipid/protein mass ratio of the various (A-I)rHDL preparations. B, HCAECs were preincubated for 16 h with the various untreated or chymase-treated apoA-I or (A-I)rHDL species (50 μg/mL, each) and then activated with tumor necrosis factor-α (TNF-α), as described in Figure 1. Nonactivated cells (control) and TNF-α-activated cells preincubated in only medium (buffer) acted as references. Vascular cell adhesion molecule-1 (VCAM-1) mRNA levels (top, fold change relative to control) and surface protein expression (middle, % of buffer) were evaluated in the TNF-α-activated cells. In a separate experiment, HCAECs that had been preincubated with the various apoA-I-containing preparations were further incubated for 1 h with fluorescently labeled THP-1cells, and the fluorescence of HCAECs-bound THP-1 cells was then measured (bottom). The fluorescence signal of the TNF-α-activated HCAECs was expressed as percentage of the basal signal from the nonactivated cells (control) which was set as 100%. Data shown in each panel represent the means±SD from 3 to 4 independent experiments performed in triplicate wells. **P<0.05; *P<0.01; #P<0.01 (untreated apoA-I-containing preparations vs buffer).
