Table 1.
Substrate scope for the nitroalkene bearing an internal alkynes with a phenyl
substituent.[a]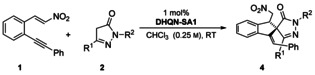
| 4 | R1 | R2 | Yield [%][b] | d.r.[c] | ee [%][d] |
|---|---|---|---|---|---|
| a | Me | Ph | 99 | 10:1 | 90 |
| b | Et | Ph | 83 | >20:1 | 93 |
| c | iPr | Ph | 74 | >20:1 | 92 |
| d | Ph | Ph | 95 | 6:1 | 91 |
| e | Me | 2-Cl-C6H4 | 73 | 10:1 | 82 |
| f | Me | 4-Me-C6H4 | 89 | 10:1 | 99 |
| g[e] | Me | Me | 59 | 20:3 | 86 |
[a] Reaction conditions: 1 (0.33 mmol), 2 (0.3 mmol), DHQN-SA1 (3 mol %), CHCl3 (1.2 mL), RT, 1–2 h. [b] Yield of 4 after flash chromatography. [c] Determined by 1H NMR spectroscopy. [d] Determined by HPLC using a chiral stationary phase. [e] The formation of small amounts of an additional diastereomer or regioisomer was detected.
