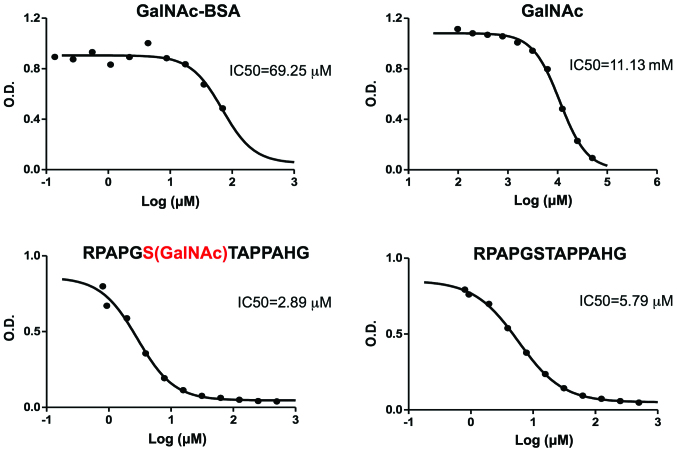
Figure 4.
16A mAb binds to both sugar and peptide parts of a MUC1 glycopeptide. The biotinylated glycopeptide, RPAPGS(GalNAc)TAPPAHG-dPEG™11-Biotin, (1 μg/ml) was bound to streptavidin-coated plates (2 μg/ml) and incubated with 16A monoclonal Ab (mAb) for 2 h. Binding of 16A was visualized by a secondary Ab (goat anti-mouse IgG) followed by colorimetric detection. To measure the inhibitory effects of competing ligands, ligands (GalNAc-BSA, GalNAc, RPAPGS(GalNAc)TAPPAHG, and RPAPGSTAPPAHG) were mixed with the 16A mAb at 0 to 500 μM for 1 h, before incubation with plate-bound glycopeptide RPAPGS(GalNAc)TAPPAHG-dPEG. Data were representative of 3 independent experiments.