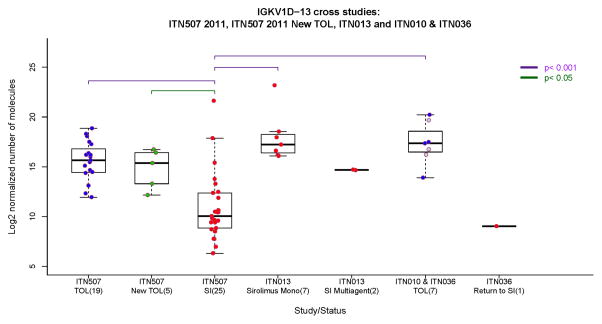
Figure 7.
Cross-sectional IGKV1D-13 expression in renal transplant recipients enrolled in multiple ITN studies. ITN507 is the tolerant renal transplant registry (TOL n=19; New-TOL n=5; SI n=25); ITN010 and 036 are the mixed chimerism trials with combined bone marrow and renal transplants. Samples from 7 operationally tolerant patients (TOL) were collected 0.33 – 7 years after discontinuation of immunosuppression (no differences in IGKV1D-13 expression were seen in relation to time, not shown). The three patients that eventually had to restart immunosuppression are indicated by light blue circles. One patient who was returned to SI (Return to SI) had their sample obtained one year after transplantation (which was 2 months after the patient rejected and was returned to immunosuppression). ITN013 is the Campath-1H followed by tacrolimus plus sirolimus trial where samples were obtained 4 years after transplantation. In ITN013, 7 patients were weaned and stable on sirolimus monotherapy (Sirolimus Mono), while 2 patients required re-institution of tacrolimus plus mycophenolate (SI Multiagent). https://www.itntrialshare.org/FACTOR_fig7.url