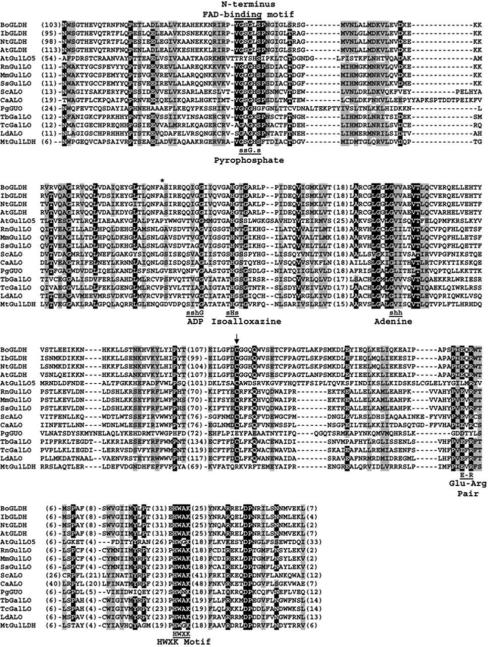
Figure 2. Multiple sequence alignment of all characterized aldonolactone oxidoreductases including Arabidopsis GulLO (AtGulLO5 or At2g46740).
Conserved residues interacting with the parts of FAD in the alignment are shown as in Fraaije et al (1998). (G, glycine; H, histidine; s, small: G, A, S, T; h, hydrophobic: I, L, V; -, any residue). Numbers in parenthesis indicate the residues present in gaps and termini. The residue indicating oxygen reactivity is marked by asterisk (*; Leferink et al. 2009b). Arrow indicates the cysteine residue known to be involved with thiol modifying agents in AtGLDH (Leferink et al. 2009d). The Glu-Arg pair identified in the catalytic site of AtGLDH (Leferink et al. 2009c) is also highlighted. The sequences used are: BoGLDH (cauliflower; Swiss-Prot: O47881), IbGLDH (sweet potato; GenBank: BAA34995), NtGLDH (tobacco; GenBank: BAB13368), AtGLDH (Arabidopsis; Swiss-Prot: Q9SU56), AtGulLO5 (Arabidopsis; GenBank: AEC10747), RnGulLO (rat; Swiss-Prot: P10867), MmGulLO (mouse; GenBank: AAR15891), SsGulLO (pig; GenBank: AAN63634), ScALO (S. cerevisiae; Swiss-Prot: P54783), CaALO (C. albicans; GenBank: AAC98913), PgGUO (P. griseoroseum; Swiss-Prot: Q671X8), TbGalLO (T. brucei; Swiss-Prot: Q57ZU1), TcGalLO (T. cruzi; Swiss-Prot: Q4DPZ5), LdALO (L. donovani; GenBank: ACV04074), MtGulLDH (M. tuberculosis; Swiss-Prot: O06804).