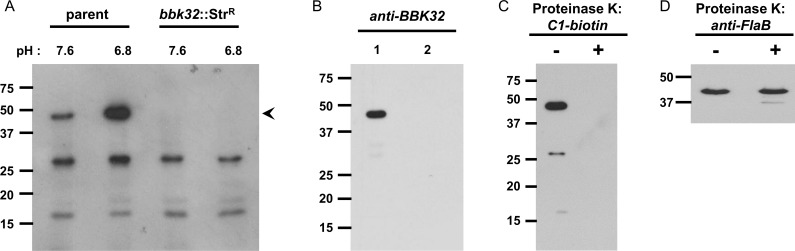
Fig 1. B. burgdorferi expresses surface-exposed proteins that bind complement C1.
Far Western Blot analysis of B. burgdorferi cell lysates probed with complement C1. (A) Biotinylated C1 was used to probe cell lysates from B. burgdorferi B31 strain ML23 grown under conventional (pH 7.6) or induced (pH 6.8) conditions. Proteins of apparent molecular weights 17, 28, and 48 kDa were capable of binding C1, while the 48-kDa band alone was inducible. Lysates from a strain lacking an intact bbk32 locus (bbk32::StrR) lacked the 48-kDa band. (B) Immunoblot analysis with anti-BBK32 demonstrated that the 48-kDa protein co-migrates with the C1 reactive species in the parent strain (lane 1) and is missing from the bbk32::StrR mutant (lane 2) providing further support that it is BBK32. Both strains shown here were grown at 37°C, pH 6.8, e.g., under inducing conditions. (C) To determine if the C1 binding proteins present in borrelial lysates were surface exposed, a proteinase K assay was employed. All three bands are absent from proteinase K treated samples. (D) The subsurface endoflagellar protein FlaB are identical between mock and protease treated cells, indicating that the B. burgdorferi cells retained structural integrity.