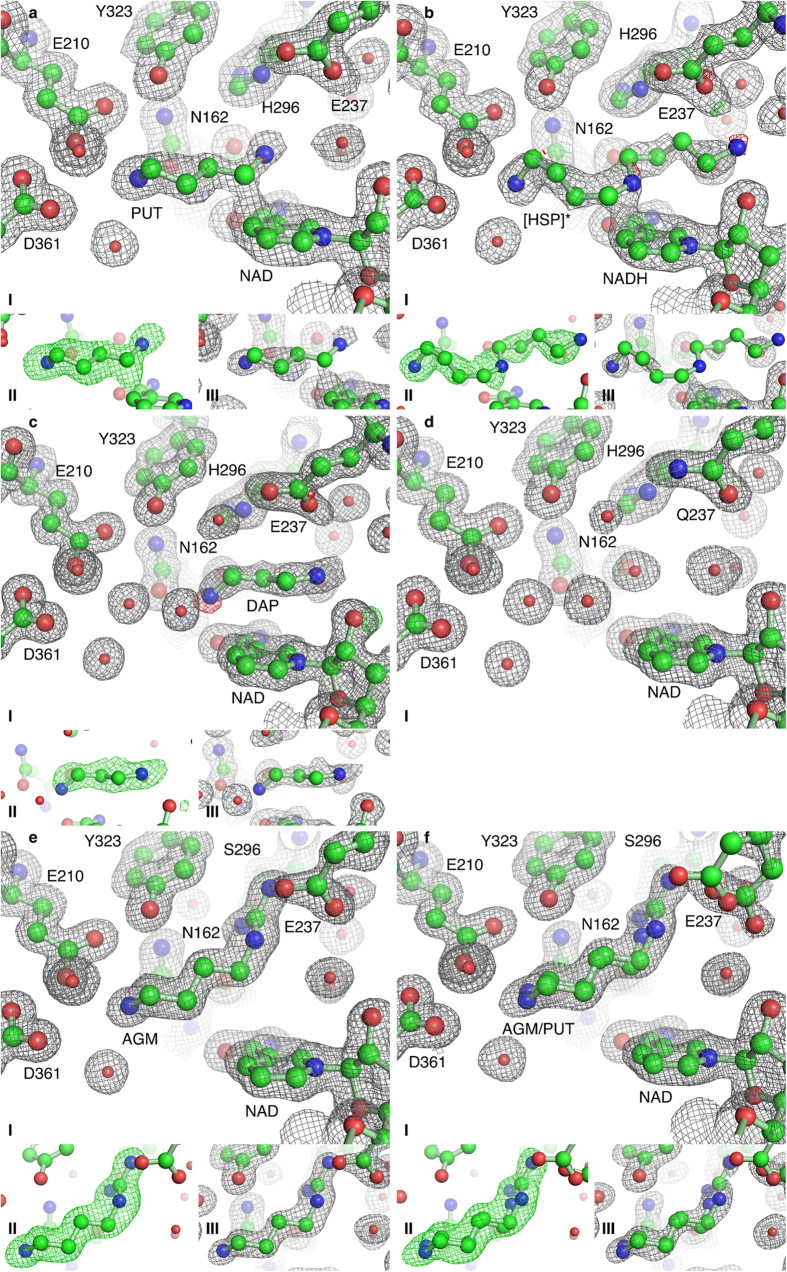
Figure 6. Active site of BvHSS and BvHSS variants E237Q and H296S with bound polyamines.
The electron density maps are shown as a mesh at a contour level of 1σ (2mFo-DFc in gray) and +/− 5σ (mFo-DFc, green/red). Electron density maps obtained from PHENIX.refine are shown in (I), and the simulated annealing (SA) ligand omit electron density (ED) maps obtained from PHENIX.composite_omit_map are shown in (II, mFo-DFc) and (III, 2mFo-DFc). Relevant residues are shown as ball-and-stick representations. (a,b) Active site of BvHSS (PDB ID: 4TVB) with bound substrates in subunit A and subunit B. The PUT bound in subunit B is shown in (a). The transition close state of the reduction to HSP bound in subunit A is shown in (b). (c) Active site of BvHSS with bound DAP (subunit A, PDB ID: 4XQE). (d) Active site of BvHSS variant E237Q (subunit B, no SA ligand omit ED maps were calculated because of the lack of bound substrate, PDB ID: 4XQG). (e,f) Active site of BvHSS variant H296S with bound AGM (e, PDB ID: 4XQE, subunit B) or with bound AGM and PUT as alternate conformations (f, PDB ID: 4XRG, subunit B). The adjustment of the occupancy values for AGM and PUT (with a constrained occupancy group per molecule to ensure equal occupancy for each atom of the respective molecule) was performed by PHENIX.refine during refinement. The calculated occupancy is in subunit A 0.56 for AGM and 0.44 for PUT and in subunit B 0.66 for AGM and 0.34 for PUT.