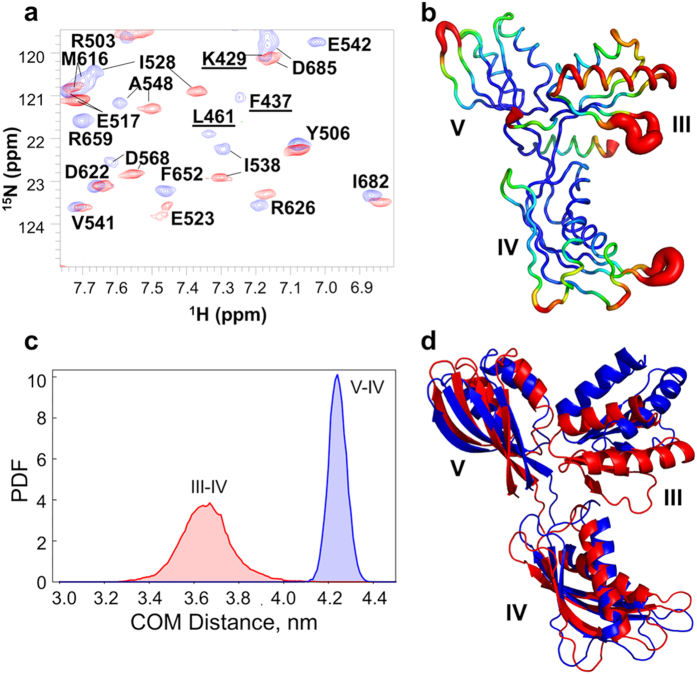
Figure 5. Domain III of EF-GC3 undergoes dynamic changes upon binding to FusB.
(a) Overlay of the 15N-TROSY-HSQC of apo EF-GC3 (blue) and EF-GC3 bound to FusB (red). Peaks from domain III are identified by underlined labels and are absent from the spectrum determined in the presence of FusB. (b) Backbone RMSF per residue determined from molecular dynamics simulations of EF-GC3. Domain III shows greater dynamic motions than domains IV and V, particularly within the first helix and β-strand. (c) Probability density functions for distance distributions between the centres of mass for domains III-IV and IV–V showing greater variation in the distances for domains III–IV, suggesting domain III is flexible. (d) The extent of motions of domain III relative to domains IV and V identified by molecular dynamics simulations. Structures are aligned on both domains IV and V, and residues in regions linking the three domains were ommited from the analysis.