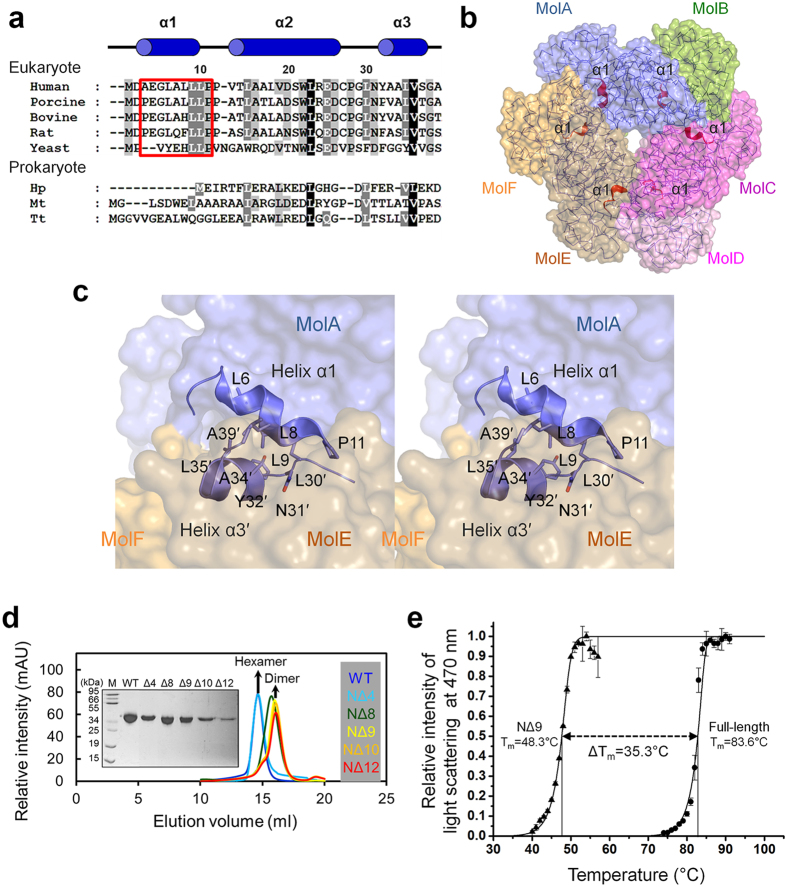
Figure 4. Involvement of helix α1 of HsQPRT in dimer-dimer interaction.
(a) Multiple sequence alignment of hexameric QPRT homologs. The database and accession code of aligned sequences are indicated in parentheses. Homo sapiens QPRT, human (Uniprot code Q15274); Sus scrofa QPRT, porcine (PDB code 4I9A); Bos taurus QPRT, bovine (NCBI code AAI02551); Rattus norvegicus QPRT, rat (NCBI code AAH88177); Saccharomyces cerevisiae QPRT, yeast (PDB code 3C2E); Helicobacter pylori QPRT, Hp (PDB code 2B7N); Mycobacterium tuberculosis QPRT, Mt (PDB code 1QPO); Thermus thermophilus QPRT, Tt (PDB code 1 × 1O). Red box indicates N-terminal helix α1. Annotation of helices α1–α3 (blue cylinders) corresponding to HsQPRT. Residue numbers are based on human protein. (b) Location of helix α1. Six α1 helices in the hexameric protein are shown as red ribbons. (c) Intermolecular interaction between helix α1 and helix α3 of the adjacent dimer (stereographic views). Residues of helix α3 in the adjacent dimer (MolE) are primed. (d) Size exclusion chromatographic analysis of HsQPRT in wild-type (blue), NΔ4 (light blue), NΔ8 (green), NΔ9 (yellow), NΔ10 (orange) and NΔ12 (red). The sample state was confirmed using SDS-PAGE. (e) Heat aggregation profile of HsQPRT. Time intervals of approximately 2 min (1 °C) are given between data points. Full-length and NΔ9 mutant proteins are labelled as circles and triangles, respectively.