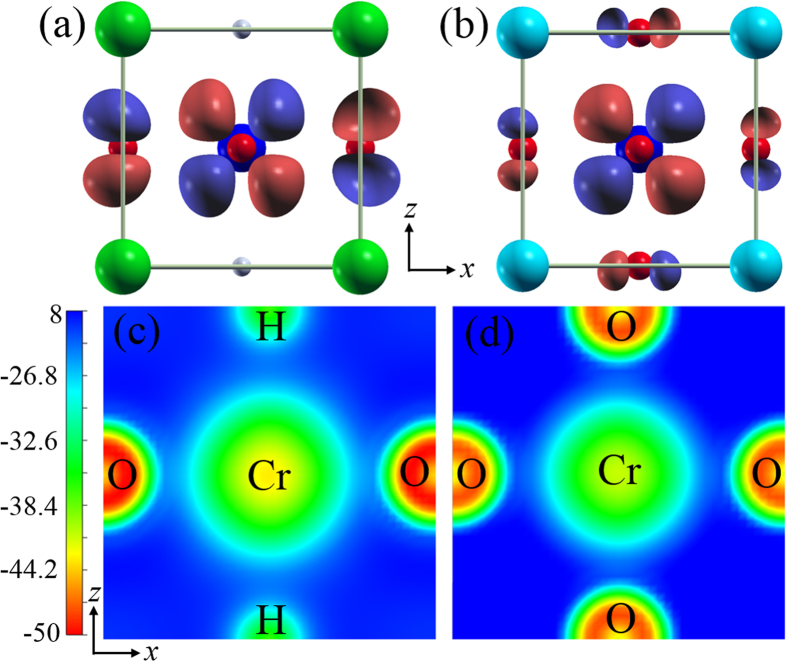
Figure 4. Isosurface plots of the Cr-dxz like MLWFs for (a) cubic SrCrO2H and (b) cubic LaCrO3.
The lobes on the O2− ion in the MLWF of SrCrO2H are bigger than those of LaCrO3, indicating that the hybridization between Cr-dxz orbital and O-pz is stronger in SrCrO2H. Contour plots of the electrostatic potential in (c) cubic SrCrO2H and (d) cubic LaCrO3, projected on the xz-plane passing through Cr, H, and O sites. The electrostatic potential along the Cr-H direction is much weaker than that along the Cr-O direction in SrCrO2H.