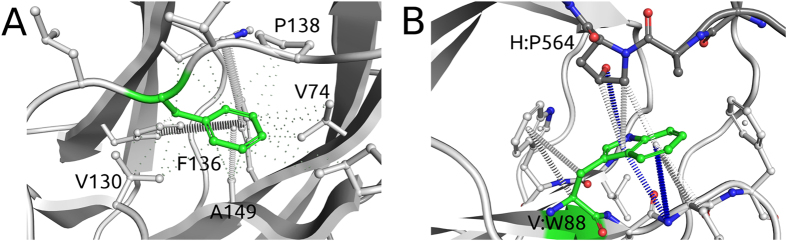
Figure 8. Noncovalent interaction networks in VHL.
Mutated residues are depicted in green. Proximal hydrophobic interactions are depicted in small dots, ring-ring interactions in grey and donor-pi interactions in blue. (Panel A) shows residue Phe136 performing a dense network of hydrophobic and ring interactions. Mutation to serine is predicted to be highly destabilizing, given the removal of a large portion of the side chain and consequent loss of interactions. Panel B shows residue Trp88, whose mutations are predicted to also affect protein-protein affinity. Trp88 establishes a network of ring interactions, as well as donor-pi interactions within its chain and with the HIF-1α peptide. Mutations to arginine or serine would disrupt these strong interactions, destabilize the region as well as the protein-protein interface, reducing affinity.