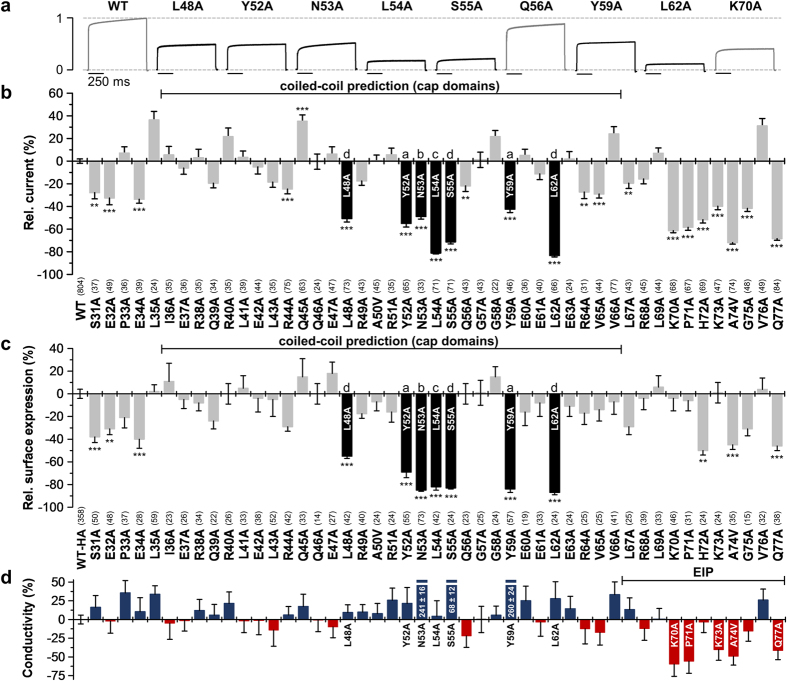
Figure 2. Alanine scan through the extracellular M1-P1 linker of human TASK-1 reveals hydrophobic residues relevant for functional surface expression.
(a) Normalized representative current traces of wild-type and mutant TASK-1 channels recorded in Xenopus oocytes by a voltage step to +40 mV. (b) Relative current amplitudes at +40 mV compared to wild-type TASK-1 (WT). Mutants within the predicted coiled-coil domain exhibiting a pronounced current amplitude reduction are highlighted in black. (c) Relative surface expression analyzed with a chemiluminescence assay of wild-type TASK-1 with an extracellular HA epitope (WT-HA) and TASK-1 mutants introduced into the WT-HA background. Mutants within the predicted coiled-coil domain showing a strong reduction in relative surface expression are highlighted in black. (b,c) **p < 0.01; ***p < 0.001. Unpaired Student’s T-Test. Significance was probed against WT or WT-HA. Data are presented as Mean ± SEM. The number of experiments are indicated in parentheses above the construct name. (d) The ‘conductivity’ of TASK-1 mutants was calculated by dividing the relative change in current amplitudes of a mutant by the relative change in surface expression of the respective mutant. Red bars indicate a loss of channel gating, due to a reduced single-channel conductance or open probability (Po). Data are presented as Mean ± SEM.