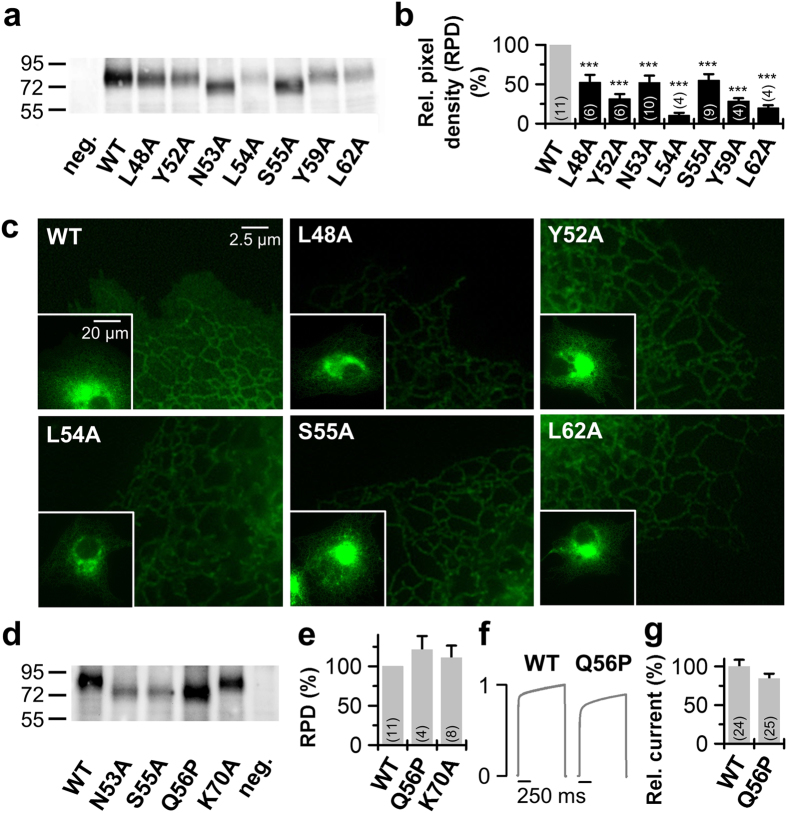
Figure 3. Protein expression of the identified TASK-1 ‘hits’.
(a) Western blot analysis of Xenopus protein lysates after injection of HA-tagged TASK-1 wild-type (WT) or indicated TASK-1 mutants using anti-HA antibodies. Samples were not treated with a reducing agent as DTT, thus the proteins appear as a dimer. (b) Analysis of the pixel intensities of the protein signals corresponding to dimeric TASK-1 from 4 to 11 independent western blots normalized to TASK-1 wild-type without DTT. Image J was used for pixel intensity analysis. RPD: relative pixel density. (c) Live cell imaging of TASK-1 mutants in HeLa cells. HeLa cells were transfected with the indicated pEGFP-TASK-1 mutants and fluorescence imaging was performed 48 h after transfection. (d) Western blot analysis of protein lysates of oocytes 48 hours after injection of HA-tagged TASK-1 wild-type (WT) or indicated TASK-1 mutants using anti-HA antibodies. Samples were not treated with a reducing agent as DTT, thus proteins appear as a dimer. (e) Analysis of the pixel intensities of the protein signals corresponding to dimeric TASK-1 from 4 to 11 independent western blots normalized to TASK-1 wild-type without DTT. Image J was used for pixel intensity analysis. (f) Current traces of TASK-1 WT compared to the Q56P mutant, preventing glycosylation. (g) Current amplitudes at +40 mV for TASK-1 (WT) and the Q56P mutant normalized to TASK-1 were plotted. (b,e,g) ***p < 0.001. Unpaired Student’s T-Test. Significance was probed against wild-type TASK-1 (WT). Data are presented as Mean ± SEM. The number of experiments are included in the bar graphs.