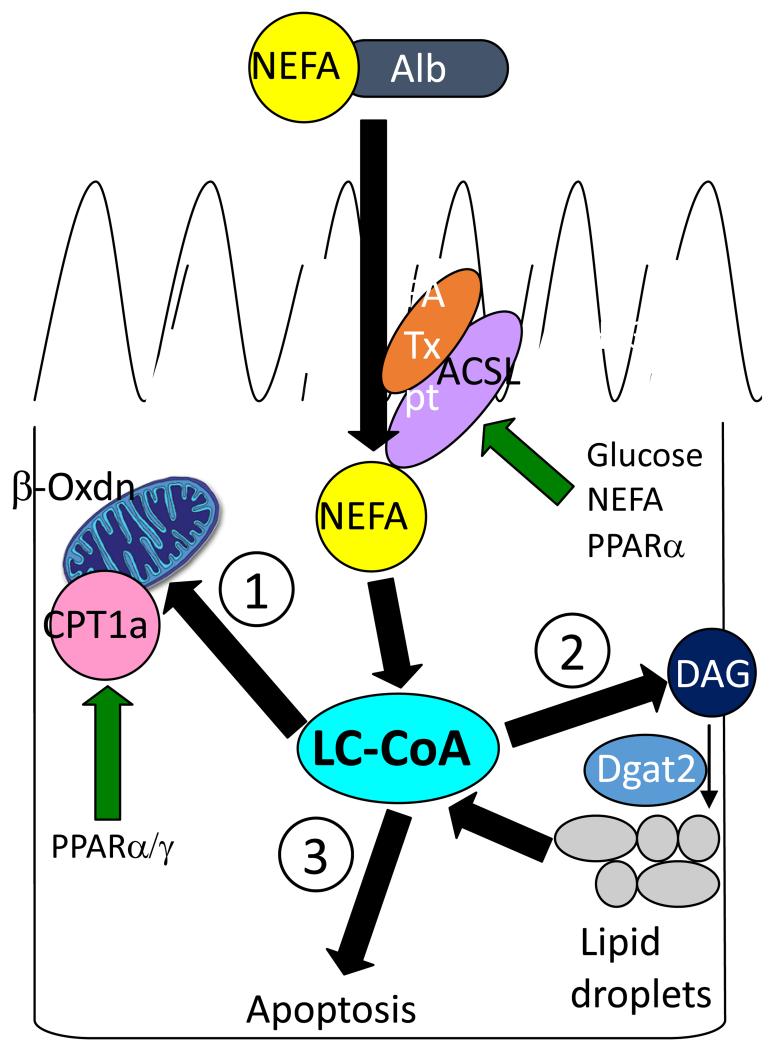
Figure 2.
Model of lipotoxicity-induced apoptosis and tubular atrophy. In progressive albuminuric renal diseases non-esterified fatty acid (NEFA) concentration is increased in proximal tubule cells through luminal reabsorption and/or lipogenesis. NEFA are rapidly converted to long chain fatty acyl CoA (LC-CoA) by long chain acyl CoA synthetases (ACSL). Preferential metabolic pathways include (1) carnitine palmitoyl transferase-1a (CPT1a)-regulated LC-CoA transport into mitochondria for β-oxidation and ATP generation or (2) conversion of diacylglycerol (DAG) to triacylglerol, which is stored in lipid droplets. If these pathways are blocked or saturated, accumulated LC-CoAs become detrimental and lead to apoptosis (3), through a variety of mechanisms, such as peroxisomal oxidation and reactive oxygen species (ROS) generation, disruption of NHE1-PI(4,5)P2 interaction-mediated cell survival, and ceramide formation.