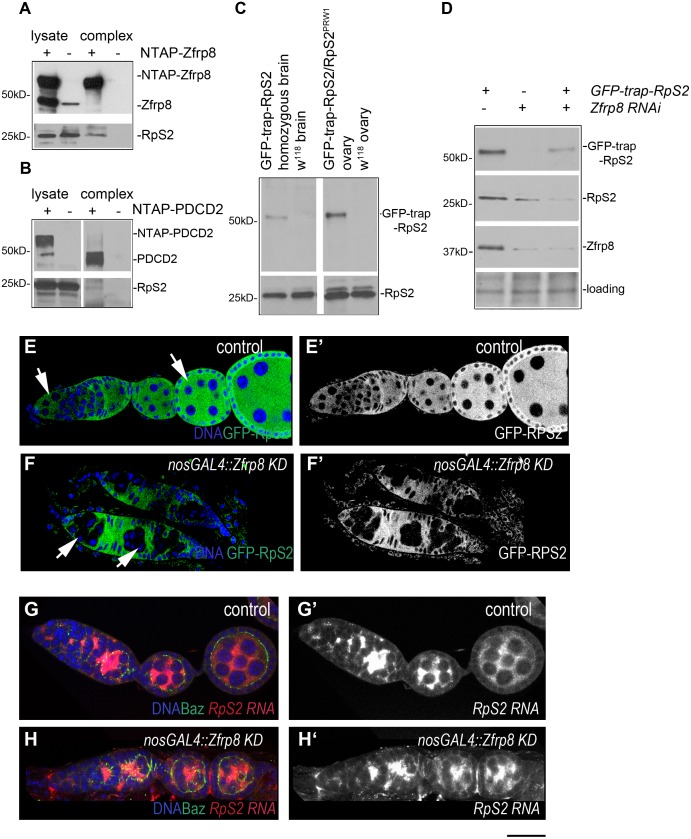
Fig 1. Zfrp8/PDCD2 complexes with RpS2 and controls its levels or stability.
(A) Western blot of the TAP-Zfrp8 complex affinity purified from Drosophila ovaries (Zfrp8null, da-GAL4/UAS-TAP-Zfrp8) was probed with anti-Zfrp8 (top) and anti-RpS2 (bottom) antibodies. Extracts from w118 flies was used as negative control. Only small portion of RpS2 was co- purified with Zfrp8, and therefore longer exposure was needed to detect RpS2 in Zfrp8 complex. (B) Western blot of the TAP-PDCD2 complex affinity purified from HEK293 cells was probed with anti-PDCD2 (top) and anti-RpS2 (bottom) antibodies. Cells transfected with vector alone (NTAP) were used as negative control. (C) GFP-trap-RpS2 homozygous flies expressed both, tagged protein (anti-GFP, top), and more abundant untagged RpS2 protein (anti-RpS2, bottom). (D) Western blot of Zfrp8 KD larval brains; both GFP-trap-RpS2 and endogenous RpS2 were reduced (lane 2 and 3) compared to that in control (lane1). The blot was probed with anti-GFP, anti-RpS2, and anti-Zfrp8 antibodies. Ponceau S was used for loading control. (E-E') GFP-trap-RpS2 protein (green) was present in germ line (arrows) and somatic ovarian cells and was mainly localized to the cytoplasm. In Zfrp8 KD germ line (F, F', arrows) GFP-trap-RPS2 was significantly reduced. (G-H') FISH with RpS2 probe (red) showed no difference in RpS2 transcript level and distribution between Zfrp8 KD (H-H') and control ovaries (G-G'). Ovaries were co-stained with DAPI (DNA, blue) and anti-Bazuka antibody (green). Size bar is 20μm.