Table 1.
Electrophile scope with linchpin 15.[a]
| |||
|---|---|---|---|
| Entry | E+ | Product | Yield |
| 1 | Mel |
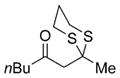
17 |
78% |
| 2 |
|
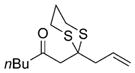
18 |
75% |
| 3 |
a)X=Br b)X=1 |
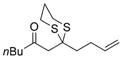
19 |
a) 59% b) 74% |
| 4 |
a)X=Br b)X=1 |
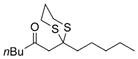 20 |
a) 52% b) 67% |
| 5 |
 a)X=Br b)X=1 |
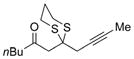 21 |
a) 55% b) 69% |
| 6 | Et3SiCl |
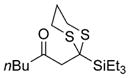 22 |
71% |
| 7 |
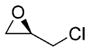 (R)-epichiorohydrin |
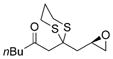 (R)-23 |
60% |
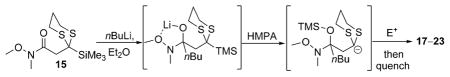
Reaction conditions: i) 15, THF, –78° C, then slow addition of nBuLi; ii) electrophile, HMPA, THF, –78° C to –30° C; iii) sat. aq. NH4Cl.
