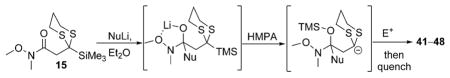
Table 3.
Preparation of cyclic systems utilizing 15.[a]
| |||||
|---|---|---|---|---|---|
| Entry | NuLi | E+ | Quench | Product | Yield |
| 1 | nBuLi |
|
A |
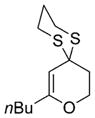
41 |
68% |
| 2 | nBuLi |
|
A |
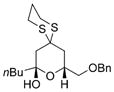
42 |
69% |
| 3 | nBuLi |

|
B |
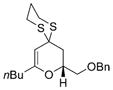 43 |
72% |
| 4 |
44 |
BnBr | B |
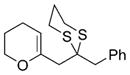 45 |
60% |
| 5 |
44 |
|
B |
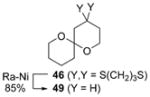
|
64% |
| 6 |
47 |

|
B |
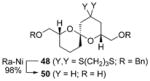
|
67% |
Reaction conditions: i) 15, Et2O, –78 ° C, then slow addition of NuLi; ii) BnBr, HMPA, Et2O, –78 ° C to –30° C; iii) quench A with sat. aq. NH4Cl or quench B with 48 % aq. HF, then NaHCO3.
