Abstract
The present study aimed to evaluate the predictive value of pelvic anatomical and clinicopathological parameters for use in the estimation of the likely technical difficulties that may be encountered when performing open rectal surgery for mid-low rectal cancer. Sixty consecutive patients, undergoing open rectal surgery for mid-low rectal cancer were recruited between June 2009 and April 2014. All of the surgical procedures conducted, were low anterior resection (LAR) or abdominoperineal resection (APR). The operations were performed by the same surgeon and surgical team. Pelvic dimensions and angles were measured using three-dimensional reconstruction of spiral computerized tomography (CT) images. Operative time and intraoperative blood loss were used as indicators of operative difficulty. The independent variables were pelvic anatomical and clinicopathological parameters, and the dependent variables were operative time and intraoperative blood loss. Univariate and multivariate analyses were performed in order to determine the predictive significance of these variables. The pelvis width was significantly wider in females than in males (P<0.05), while the sacrococcygeal bending degree was significantly greater in males than in females (P<0.05). No significant difference were detected between the pelvis depth of females and males (P>0.05). Multivariate analyses showed that body mass index (BMI), tumor height, lymph node metastasis, anteroposterior diameter of the pelvic inlet, anteroposterior diameter of the pelvic outlet, height of the pubic symphysis, the sacrococcygeal distance, sacrococcygeal-pubic angle and diameter of the upper pubis to the coccyx were the main factors affecting the operative time (all P<0.05), while the maximum diameter of the tumor was the primary factor affecting intraoperative blood loss (P<0.05). Between the two procedures, the clinicopathological parameters appeared to be more valuable for predicting difficulty in LAR, in which operative time was associated with tumor height and tumor staging (RC2=0.312; P<0.001). By contrast, the pelvic anatomical parameters appeared to be more valuable predictors of variation in APR, in which intraoperative blood loss was associated with the anteroposterior diameter of the mid-pelvis, the anteroposterior diameter of the pelvic outlet, the interspinous diameter, the depth of the sacral curvature and the sacropubic distance (RC2=0.608; P=0.002). BMI, tumor height and the maximum diameter of the tumor may be used to predict the operative difficulty in performing open rectal surgery for mid-low rectal cancer. In addition to the associated clinicopathological parameters, wider, shallower and less curved pelvises may make the greatest contribution to reducing operative time and intraoperative blood loss. Operative difficulty is likely to be increased in deeper and narrower pelvises, or in those with greater sacrococcygeal curvature.
Keywords: computerized tomography, rectal cancer, pelvimetry, clinicopathological parameters, operative time, intraoperative blood loss, three-dimensional reconstruction
Introduction
Rectal cancer is one of the commonest causes of cancer-related mortality worldwide, and its incidence is increasing year-on-year. Surgery remains the mainstay of treatment for rectal cancer. The aims of surgery for rectal cancer are to achieve cure and to avoid locoregional recurrence (1). The principle of total mesorectal excision (TME) has been widely applied, which has significantly reduced the local recurrence rate for rectal cancer (1,2). However, the success of TME for the treatment of rectal cancer is influenced by the surgeon's experience, in addition to patient anatomical and clinical factors (3–5). The patient's pelvic anatomical factors are important, as TME is performed in the narrow and funnel-shaped pelvic cavity, which makes access and visualization in the deep pelvis, difficult. It is challenging to maintain a clear surgical field, to recognize precise anatomy, and to accurately perform rectal mobilization and excision (6).
Surgeons are aware that the female pelvis is, in general, more accessible than the male pelvis when conducting open rectal surgery for mid-low rectal cancer. In general, female pelvises are wider and shallower than male pelvises. However, there is considerable variation and overlap between the sexes (7). At present, there is no consensus on how pelvic diameter and angle influence the technical difficulty of performing open rectal surgery for mid-low rectal cancer. In the present study, operative time and intraoperative blood loss were selected as indicators of operative difficulty, as they have been objectively validated as such in the literature (6,8,9).
The aim of the present study is to evaluate the predictive value of clinicopathological and pelvic anatomical parameters in estimating the operative time and intraoperative blood loss when performing open rectal surgery for mid-low rectal cancer, in order to assist colorectal surgeons in the identification of potentially difficult rectal resections and in the design of appropriate preoperative plans.
Patients and methods
Patients and surgical procedures
Sixty consecutive patients, who underwent low anterior resection (LAR) with double-stapling technique (DST) anastomosis or abdominoperineal resection (APR) for mid-low rectal cancer located within 10 cm of the anal verge, were recruited between June 2009 and April 2014. The distance from the anal verge to the lower margin of the tumors (tumor height) was measured by digital rectal examination and/or colonoscopy. All cases were confirmed as adenocarcinoma by biopsy prior to surgery. Operations were performed by the same surgeon and surgical team, who were experienced in TME techniques, at the Department of Surgery of Wenzhou Central Hospital (Wenzhou, China).
Patients who had undergone previous abdominal surgery through a laparotomy, who had a history of pelvic fracture, who had received neoadjuvant chemoradiotherapy and those with locally recurrent disease were excluded from the present study. Patients were also excluded if tumors had infiltrated to the adjacent organs, or had metastasized to the lateral pelvic wall lymph nodes or distant sites. The preoperative clinical stage of rectal cancer was assessed by contrast-enhanced computerized tomography (CT).
Data on age, gender, body mass index (BMI), the maximum diameter of the tumor, tumor height, tumor invasive depth, lymph node metastasis, tumor staging, operative time and intraoperative blood loss were collected retrospectively. Tumors were staged according to the 7th tumor-node-metastasis (TNM) classification of the International Union against Cancer (UICC) (10), on the basis of the histological findings of the surgical specimens. Written informed consent for participation was obtained from participants, or from their parent or guardian. This study was approved by the Institutional Review Board of Wenzhou Central Hospital.
Pelvimetry
All patients underwent contrast-enhanced abdominopelvic CT. Three-dimensional reconstruction of the pelvis was performed on a workstation, using the syngo CT imaging program (version VA44A; Siemens AG, Muenchen, Germany) with a scanning slice thickness of 1.0 mm and interslice interval of 1.0 mm. Pelvic dimensions and angles were obtained using mid-sagittal and axial sections of the pelvis.
All measurements were made by a single observer who was blinded to all clinical information. The following fourteen pelvic parameters, including twelve dimensions and two angles, were measured:
-
1.
Anteroposterior diameter of the pelvic inlet (AB): A line from the superior median aspect of the pubic symphysis to the sacral promontory.
-
2.
Anteroposterior diameter of the mid-pelvis (CD): A line from the inferior median aspect of the pubic symphysis to the sacrococcygeal junction.
-
3.
Anteroposterior diameter of the pelvic outlet (CE): A line from the inferior median aspect of the pubic symphysis to the tip of the coccyx.
-
4.
The interspinous diameter (LM).
-
5.
The intertuberous diameter (NO).
-
6.
The height of the pubic symphysis (AC).
-
7.
The sacrococcygeal distance (BE): Distance from the sacral promontory to the tip of the coccyx.
-
8.
The sacral distance (BD): Distance from the sacral promontory to the sacrococcygeal junction.
-
9.
Sacrococcygeal-pubic angle (α): The angle between an extension of the line forming the anteroposterior diameter of the pelvic inlet and that of the anteroposterior diameter of the pelvic outlet.
-
10.
Sacropubic angle (β): The angle between an extension of the line forming the anteroposterior diameter of the pelvic inlet and that of the anteroposterior diameter of the mid-pelvis.
-
11.
The depth of the sacrococcygeal curvature (FI): A perpendicular line from the deepest portion of the sacrococcygeal hollow to the sacrococcygeal distance.
-
12.
The depth of the sacral curvature (FH): A perpendicular line from the deepest portion of the sacral hollow to the sacral distance line.
-
13.
Diameter of the upper pubis to the coccyx (AE): A line from the superior median aspect of the pubic symphysis to the tip of the coccyx.
-
14.
Sacropubic distance (FG): A perpendicular line from the deepest portion of the sacrococcygeal hollow to the height of the pubic symphysis or an extension of this line.
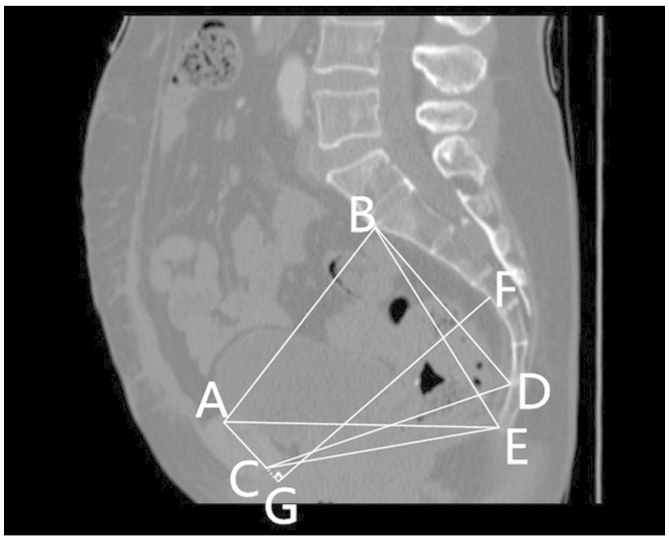
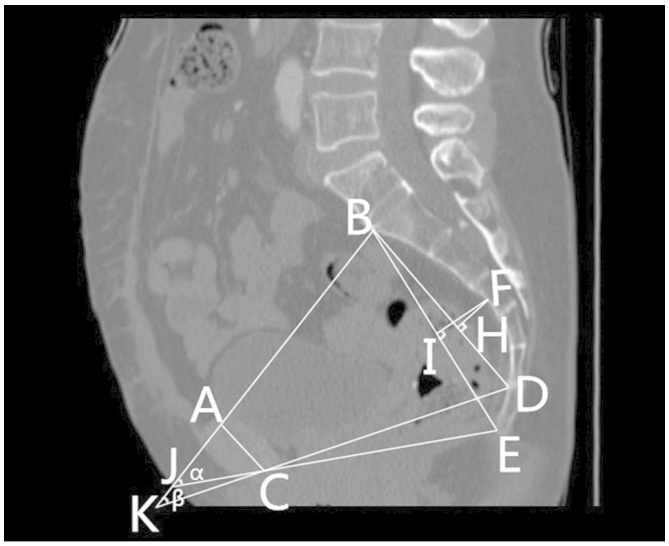
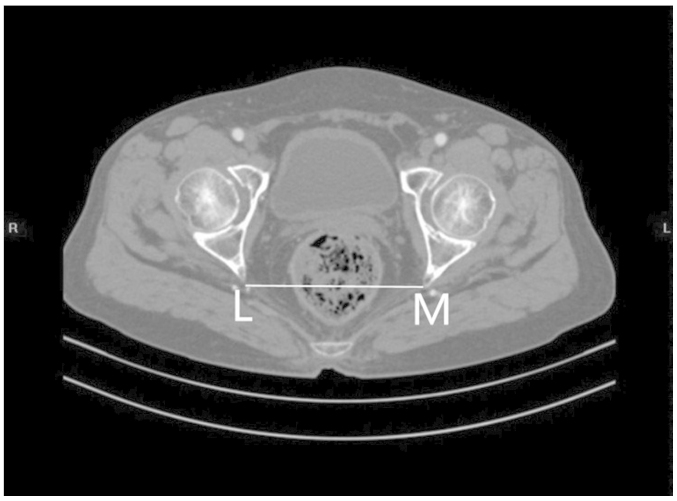
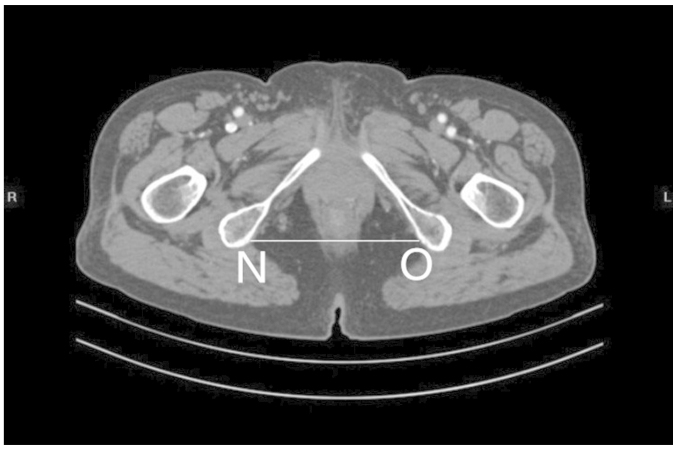
Figs. 1 and 2 outline the mid-sagittal view of the pelvis in a female patient. Fig. 3 outlines the axial section, showing the interspinous diameter of the mid-pelvis. Fig. 4 outlines the axial section, showing the intertuberous diameter of the pelvic outlet. The relevant measurements are indicated in Figs. 1–4. Assessment of intraobserver error was conducted as detailed in the statistics section.
Figure 1.
Mid-sagittal view of the pelvis in a female patient, indicating pelvic dimensions. (AB) Anteroposterior diameter of the pelvic inlet. (CD) Anteroposterior diameter of the mid-pelvis. (CE) Anteroposterior diameter of the pelvic outlet. (AC) Height of the pubic symphysis. (BE) Sacrococcygeal distance. (BD) Sacral distance. (AE) Diameter of the upper pubis to the coccyx. (FG) Sacrcopubic distance. (A) The superior median aspect of the pubic symphysis. (B) The anterior median aspect of the sacral promontory. (C) The inferior median aspect of the pubic symphysis. (D) The anterior median aspect of the sacrococcygeal junction. (E) The inferior median aspect of the tip of the coccyx. (F) The deepest portion of the sacral hollow or sacrococcygeal hollow. (G) A point of the perpendicular line from the deepest portion of the sacrococcygeal hollow to the height of the pubic symphysis or an extension of this line.
Figure 2.
Mid-sagittal view of the pelvis in a female patient, indicating the (FI) depth of the sacrococcygeal curvature, (FH) the depth of the sacral curvature and the (α) sacrococcygeal-pubic and (β) sacropubic pelvic angles. (A) The superior median aspect of the pubic symphysis. (B) The anterior median aspect of the sacral promontory. (C) The inferior median aspect of the pubic symphysis. (D) The anterior median aspect of the sacrococcygeal junction. (E) The inferior median aspect of the tip of the coccyx. (F) The deepest portion of the sacral hollow or sacrococcygeal hollow. (H) A point of the perpendicular line from the deepest portion of the sacral hollow to the sacral distance line. (I) A point of the perpendicular line from the deepest portion of the sacrococcygeal hollow to the sacrococcygeal distance. (J) The point between an extension of the line forming the anteroposterior diameter of the pelvic inlet and that of the anteroposterior diameter of the pelvic outlet. (K) The point between an extension of the line forming the anteroposterior diameter of the pelvic inlet and that of the anteroposterior diameter of the mid-pelvis.
Figure 3.
Axial section showing the interspinous diameter (LM) of the mid-pelvis.
Figure 4.
Axial section showing the intertuberous diameter (NO) of the pelvic outlet.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are shown as the mean ± standard deviation or medians (range), as appropriate. Operative time and intraoperative blood loss were used as indicators of operative difficulty. Operative time and intraoperative blood loss were defined as dependent variables, and pelvic anatomical and clinicopathological parameters were defined as independent variables. Determination of statistically significant factors was made by univariate and multivariate analysis.
Where appropriate, an independent samples t-test was applied to analyze differences in pelvic anatomical parameters between males and females. Pearson's product-moment correlation coefficient or Spearman's rank correlation coefficient were applied to analyze associations between patients' pelvic anatomical and clinicopathological parameters, and operative difficulty. Multivariate analysis was performed using a multiple linear regression model with a backward method. P≤0.05 was considered to indicate a statistically significant difference.
In order to assess intraobserver variation, measurements of the pelvic anatomical parameters of 20 patients were repeated following an interval of 4 weeks, with the observer blinded to the initial results. A paired-samples t-test was then applied to the data. Intraobserver variation was calculated using Pearson's product-moment correlation coefficient. The lowest value obtained was 0.991. The two sets of measurements were highly correlated (P<0.001), indicating that measurements were reproducible and accurate.
Results
Patients' clinicopathological data and pelvic anatomical parameters are summarized in Tables I and II, respectively. The nine pelvic parameters, in which there were significant differences between the sexes, were anteroposterior diameter of the pelvic inlet, anteroposterior diameter of the mid-pelvis, anteroposterior diameter of the pelvic outlet, the interspinous diameter, the intertuberous diameter, sacrococcygeal-pubic angle, the depth of the sacrococcygeal curvature, diameter of the upper pubis to the coccyx and sacropubic distance (P<0.05). No significant differences were detected between the sexes in the remaining five pelvic parameters; namely, the height of the pubic symphysis, the sacrococcygeal distance, the sacral distance, sacropubic angle and the depth of the sacral curvature (P>0.05).
Table I.
Clinicopathological data (n=60).
| Variable | Value |
|---|---|
| Gender, n (male:female) | 38:22 |
| Age, years (range) | 65.3±13.0a (29–85) |
| Body mass index, kg/m2 (range) | 21.31±3.07a (16.2–30.8) |
| Tumor height, cm (range) | 5.9±2.0a (2.0–10.0) |
| Maximum diameter of the tumor, cm (range) | 4.0b (3.0–8.0) |
| Operative time, min (range) | 161.3±41.9a (75–275) |
| Intraoperative blood loss, ml (range) | 50b (50–600) |
| Surgical procedure, n | |
| Abdominoperineal resection | 40 |
| Low anterior resection | 20 |
| Tumor invasive depth, n | |
| T1 | 3 |
| T2 | 16 |
| T3 | 28 |
| T4 | 13 |
| Lymph node metastasis, n | |
| N0 | 25 |
| N1 | 23 |
| N2 | 12 |
| Tumor stage, n | |
| I | 14 |
| II | 11 |
| III | 35 |
mean ± standard deviation,
median.
Table II.
Pelvic anatomical parameters (n=60).
| Total (n=60) | Male (n=38) | Female (n=22) | P-value | |
|---|---|---|---|---|
| Anteroposterior diameter of the pelvic inlet, mm | 110.31±12.10 | 105.22±8.78 | 119.10±12.13 | <0.001 |
| Anteroposterior diameter of the mid-pelvis, mm | 111.40±8.44 | 107.96±6.74 | 117.34±7.85 | <0.001 |
| Anteroposterior diameter of the pelvic outlet, mm | 88.92±8.62 | 85.54±6.37 | 94.76±9.00 | <0.001 |
| Interspinous diameter, mm | 98.58±10.23 | 93.24±7.32 | 107.79±7.68 | <0.001 |
| Intertuberous diameter, mm | 98.46±14.25 | 91.28±9.52 | 110.87±12.50 | <0.001 |
| Height of pubic symphysis, mm | 36.73±3.50 | 37.36±3.38 | 35.65±3.53 | 0.068 |
| Sacrococcygeal distance, mm | 121.49±13.80 | 122.22±14.17 | 120.24±13.36 | 0.595 |
| Sacral distance, mm | 107.09±10.52 | 107.37±10.37 | 106.62±11.00 | 0.794 |
| Sacrococcygeal-pubic angle, ° | 51.21±8.76 | 53.61±8.81 | 47.04±7.07 | 0.003 |
| Sacropubic angle, ° | 36.98±6.41 | 38.00±6.75 | 35.21±5.46 | 0.104 |
| Depth of the sacrococcygeal curvature, mm | 37.98±5.38 | 39.20±4.81 | 35.87±5.76 | 0.020 |
| Depth of the sacral curvature, mm | 20.78±5.05 | 21.43±5.28 | 19.66±4.52 | 0.177 |
| Diameter of the upper pubis to the coccyx, mm | 113.17±8.54 | 110.91±6.68 | 117.08±10.06 | 0.015 |
| Sacropubic distance, mm | 123.13±10.15 | 120.17±6.82 | 128.24±12.81 | 0.011 |
All data are presented as the mean ± standard deviation.
Univariate analyses of the association between patients' clinicopathological and pelvic anatomical parameters, and operative difficulty are summarized in Table III. Multivariate analyses of the associations between patients' clinicopathological and pelvic anatomical parameters, and operative difficulty are summarized in Tables IV–VIII. Univariate analysis showed that BMI (P=0.02), tumor height (P=0.02), lymph node metastasis (P=0.04) and tumor staging (P=0.03) were significantly associated with operating time. However, no significant associations were detected between gender, age, the maximum diameter of the tumor, tumor invasive depth or pelvic anatomical parameters, and operating time (P>0.05). Univariate analysis showed that the maximum diameter of the tumor (P=0.05) was the only clinicopathological parameter that was significantly associated with intraoperative blood loss, while no association was detected between the remaining clinicopathological parameters or the pelvic anatomical parameters, and intraoperative blood loss (P>0.05).
Table III.
Correlations between clinicopathological and pelvic anatomical parameters, and operative difficulty.
| Correlation with operative time | Correlation with intraoperative blood loss | |||
|---|---|---|---|---|
| Variable | Correlation | P-value | Correlation | P-value |
| Gender | 0.115 | 0.38 | 0.055 | 0.67 |
| Age | −0.142a | 0.28 | 0.124 | 0.35 |
| Body mass index | 0.310a | 0.02 | −0.149 | 0.25 |
| Tumor height | −0.300a | 0.02 | 0.059 | 0.65 |
| Maximum diameter of the tumor | −0.117 | 0.37 | 0.253 | 0.05 |
| Tumor invasive depth | −0.031 | 0.81 | −0.005 | 0.97 |
| Lymph node metastasis | −0.262 | 0.04 | −0.012 | 0.93 |
| Tumor stage | −0.284 | 0.03 | 0.073 | 0.58 |
| Anteroposterior diameter of the pelvic inlet | 0.014a | 0.92 | 0.043 | 0.74 |
| Anteroposterior diameter of the mid-pelvis | −0.129a | 0.32 | −0.027 | 0.84 |
| Anteroposterior diameter of the pelvic outlet | −0.107a | 0.41 | −0.125 | 0.34 |
| Interspinous diameter | −0.090a | 0.49 | −0.015 | 0.91 |
| Intertuberous diameter | −0.060a | 0.65 | 0.037 | 0.78 |
| Height of pubic symphysis | 0.001a | 0.99 | 0.219 | 0.09 |
| Sacrococcygeal distance | −0.013a | 0.92 | −0.041 | 0.76 |
| Sacral distance | −0.103a | 0.44 | −0.047 | 0.72 |
| Sacrococcygeal-pubic angle | 0.039a | 0.77 | −0.019 | 0.89 |
| Sacropubic angle | −0.003a | 0.98 | −0.152 | 0.25 |
| Depth of the sacrococcygeal curvature | 0.048a | 0.72 | 0.188 | 0.15 |
| Depth of the sacral curvature | 0.104a | 0.43 | 0.024 | 0.85 |
| Diameter of the upper pubis to the coccyx | −0.106a | 0.42 | −0.179 | 0.17 |
| Sacropubic distance | −0.033a | 0.80 | 0.093 | 0.48 |
P≤0.05 was considered statistically significant in univariate analysis. Bold font indicates P≤0.05. Univariate analysis was performed using Pearson's product-moment correlation coefficient or Spearman's rank correlation coefficient when appropriate.
Pearson's product-moment correlation coefficient.
Table IV.
Multivariate analysis in the final backward linear regression model of clinicopathological parameters and operative time.
| Unstandardized coefficient | Standardized coefficient | ||||
|---|---|---|---|---|---|
| Model | B | SE | β | t | P-value |
| (Constant) | 132.295 | 38.672 | 3.421 | 0.001 | |
| Body mass index | 3.456 | 1.603 | 0.253 | 2.156 | 0.035 |
| Tumor height | −5.613 | 2.383 | −0.273 | −2.355 | 0.022 |
| Lymph node metastasis | −15.069 | 6.466 | −0.274 | −2.330 | 0.023 |
R=0.500, R2=0.250, RC2=0.210, F=6.235; Model utility test, P=0.001. n=60. SE, standard error.
Table VIII.
Multivariate analysis of the association between pelvic anatomical parameters and intraoperative blood loss in the abdominoperineal resection group.
| Unstandardized coefficient | Standardized coefficient | ||||
|---|---|---|---|---|---|
| Model | B | SE | β | t | P-value |
| (Constant) | 229.465 | 214.829 | 1.068 | 0.304 | |
| Anteroposterior diameter of the mid-pelvis | 22.429 | 4.322 | 2.498 | 5.189 | <0.001 |
| Anteroposterior diameter of the pelvic outlet | −14.673 | 2.908 | −1.239 | −5.045 | <0.001 |
| Interspinous diameter | −5.594 | 1.600 | −0.619 | −3.495 | 0.004 |
| Depth of the sacrococcygeal curvature | 13.276 | 4.510 | 0.747 | 2.944 | 0.011 |
| Sacropubic distance | −8.840 | 2.767 | −1.004 | −3.195 | 0.006 |
R=0.843, R2=0.711, RC2=0.608, F=6.904, Model utility test: P=0.002. SE, standard error.
In the whole group, multivariate analysis demonstrated that predictive factors for operating time included BMI, tumor height, lymph node metastasis, anteroposterior diameter of the pelvic inlet, anteroposterior diameter of the pelvic outlet, the height of pubic symphysis, the sacrococcygeal distance, sacrococcygeal-pubic angle and the diameter of the upper pubis to the coccyx (all P<0.05), while the only predictive factor for intraoperative blood loss was the maximum diameter of the tumor (P<0.05).
Subgroup analyses may provide more specific information, and multivariate analysis of the association between clinicopathological parameters and operative time in the LAR group, showed that predictive factors for operative time included tumor height and tumor stage (adjusted coefficient of determination of the regression equation, RC2=0.312; P<0.001). Multivariate analysis of the association between pelvic anatomical parameters and intraoperative blood loss in the APR group demonstrated that predictive factors for intraoperative blood loss included anteroposterior diameter of the mid-pelvis, anteroposterior diameter of the pelvic outlet, the interspinous diameter, the depth of the sacral curvature and the sacropubic distance (RC2=0.608; P=0.002).
Discussion
Patients with rectal cancer usually require spiral CT enhanced scanning for preoperative staging assessment. Spiral CT has high sensitivity and specificity, and a low radiation dose, and is also relatively inexpensive compared with magnetic resonance imaging. Therefore CT pelvimetry may be used conveniently in patients with rectal cancer. CT pelvimetry is also an accurate and reliable technique for obtaining pelvimetric measurements, which has been utilized in patients with rectal cancer for a number of years (6,11–13).
Colorectal surgeons recognize that the female pelvis is generally more accessible than the male pelvis, in terms of conducting open rectal surgery for mid-low rectal cancer. In the present study, nine pelvic parameters exhibited significant differences between the sexes; namely, anteroposterior diameter of the pelvic inlet, anteroposterior diameter of the mid-pelvis, anteroposterior diameter of the pelvic outlet, the interspinous diameter, the intertuberous diameter, sacrococcygeal-pubic angle, the depth of the sacrococcygeal curvature, diameter of the upper pubis to the coccyx and sacropubic distance (P<0.05). These pelvic parameters, with the exception of the sacrococcygeal-pubic angle and the depth of the sacrococcygeal curvature, represent the pelvic width, and were greater in females. By contrast, the depth of the sacrococcygeal curvature represents the sacrococcygeal bending degree, and was greater in the male pelvis. The sacrococcygeal-pubic angle indicates sacrococcygeal arc length and bending degree; in general, the larger the sacrococcygeal-pubic angle, the longer the sacrococcygeal arc length and the smaller the sacrococcygeal bending degree, in individuals of a similiar height. Usually, the sacrococcygeal-pubic angle is greater in the male pelvis than the female pelvis. The remaining five pelvic parameters measured in the present study, exhibited no significant differences between the sexes; namely, the height of the pubic symphysis, the sacrococcygeal distance, the sacral distance, sacropubic angle and the depth of the sacral curvature (P>0.05). The height of the pubic symphysis, the sacrococcygeal distance and the sacral distance, represent the pelvic depth; the depth of the sacral curvature represents the sacral bending degree; and the sacropubic angle indicates sacral arc length and sacral bending degree, as well as the distance between pubis and the sacrum, which exhibited a greater overlap between the sexes, suggesting that for these factors, the measurements themselves may be a more useful predictor of difficulty than gender alone, as previously mentioned by Killeen et al (8).
In the present study, multivariate analyses demonstrated that higher BMI, lower tumor height, fewer lymph node metastasis, shorter anteroposterior diameter of the pelvic inlet, shorter anteroposterior diameter of the pelvic outlet, shorter height of the pubic symphysis, longer sacrococcygeal distance, smaller sacrococcygeal-pubic angle and longer diameter of the upper pubis to the coccyx, were significantly associated with a longer operative time (all P<0.05), while a larger maximum diameter of the tumor was significantly associated with increased intraoperative blood loss (P<0.05). Between the two procedures, the clinicopathological parameters appeared to have greater predictive value in the LAR group, in which lower tumor height and lower tumor staging were significantly associated with longer operative time (RC2=0.312; P<0.001). By contrast, the pelvic anatomical parameters appeared to be more valuable predictors in the APR group, in which longer anteroposterior diameter of the mid-pelvis, shorter anteroposterior diameter of the pelvic outlet, shorter interspinous diameter, longer depth of the sacral curvature and shorter sacropubic distance were significantly associated with increased intraoperative blood loss (RC2=0.608; P=0.002).
The present findings suggest that BMI, tumor height and the maximum diameter of the tumor may be valuable for predicting operative difficulty encountered during open rectal surgery for mid-low rectal cancer, which is in accordance with previous studies (6,12,14). A higher BMI is associated with greater mesorectal volume, which restricts the pelvic working space during surgical procedures. Furthermore, a larger maximum diameter of the tumor reflects larger tumor volume, which also restricts the pelvic working space (6). The pelvic working space will also be affected by tumor height from the anal verge. Particularly in certain patients with lower rectal cancer, it is difficult to expose the deep pelvic structures to obtain an adequate working space. In addition, the present findings indicated that fewer lymph node metastases and lower tumor stage may be significantly associated with longer operative time. A possible explanation for this unusual finding, is that thin patients with rectal cancer are more prone to lymph node metastasis and intestinal wall invasion, compared with obese patients. Since the findings also showed a correlation between higher BMI and longer operative time, this group may therefore include those with fewer metastases. It has been reported that the mesorectum presents a considerable obstacle to the growth of cancer (15). The volume of perirectal fatty tissue is likely to be smaller in lean patients than in obese patients. A small volume of perirectal fatty tissue may therefore contribute to early tumor infiltration of the pelvic wall and/or adjacent organs (16), meaning that this parameter may be negatively correlated with operative time for a similar reason to that of lymph node metastasis.
The present findings concerning the predictive value of pelvic anatomical features, may, in part, be explained by the fact that open rectal surgery for mid-low rectal cancer is easier to complete in wider, shallower and less curved pelvises, although the influence of the height of the pubic symphysis, diameter of the upper pubis to the coccyx and anteroposterior diameter of the mid-pelvis, is more difficult to be rationalize. A possible reason for the effect of these factors, is that the sample size is relatively small. Therefore, studies with larger sample sizes need to be conducted for more definite results in future. In view of the results of the multivariate analyses in the APR subgroup, demonstrating the predictive value of pelvic anatomical parameters, it is more difficult for surgeons to gain entry into the presacral space by sharp dissection in narrower and more curved concave pelvises. This is due to the fact that any inaccuracy may lead to deviation from the correct anatomical level (‘holy plane’), and the avascular area, resulting in severe presacral hemorrhage. Therefore, such cases should be performed by highly experienced colorectal surgeons in order to reduce the risk of serious complications and recurrence in patients with positive margins.
The present findings indicate that BMI, tumor height and the maximum diameter of the tumor may be used to predict operative difficulty in performing open rectal surgery for mid-low rectal cancer. Furthermore, the associated clinicopathological parameters, and wider, shallower and less curved pelvises may make the greatest contribution to reducing operating times and intraoperative blood loss. Operative difficulty is likely to be increased in deeper and narrower pelvises, or in those with larger sacrococcygeal curvature.
Table V.
Multivariate analysis in the final backward linear regression model of pelvic anatomical parameters and operative time.
| Unstandardized coefficient | Standardized coefficient | ||||
|---|---|---|---|---|---|
| Model | B | SE | β | t | P-value |
| (Constant) | 485.547 | 121.961 | 3.981 | <0.001 | |
| Anteroposterior diameter of the pelvic inlet | −2.387 | 0.893 | −0.749 | −2.673 | 0.010 |
| Anteroposterior diameter of the pelvic outlet | −5.265 | 1.727 | −1.177 | −3.048 | 0.004 |
| Height of pubic symphysis | −7.103 | 2.168 | −0.645 | −3.276 | 0.002 |
| Sacrococcygeal distance | 4.075 | 1.534 | 1.458 | 2.657 | 0.010 |
| Sacrococcygeal-pubic angle | −7.006 | 2.273 | −1.590 | −3.082 | 0.003 |
| Diameter of the upper pubis to the coccyx | 4.707 | 1.587 | 1.043 | 2.966 | 0.005 |
R=0.467, R2=0.218, RC2=0.130, F=2.463; Model utility test, P=0.036. n=60. SE, standard error.
Table VI.
Multivariate analysis of the association between clinicopathological parameters and operative time in the low anterior resection group.
| Unstandardized coefficient | Standardized coefficient | ||||
|---|---|---|---|---|---|
| Model | B | SE | β | t | P-value |
| (Constant) | 287.884 | 29.880 | 9.635 | <0.001 | |
| Tumor height | −11.250 | 3.437 | −0.435 | −3.273 | 0.002 |
| Tumor stage | −21.935 | 7.014 | −0.416 | −3.127 | 0.003 |
R=0.589, R2=0.347, RC2=0.312, F=9.829; Model utility test, P<0.001. SE, standard error.
Table VII.
Multivariate analysis in the final backward linear regression model of clinicopathological parameters and intraoperative blood loss.
| Unstandardized coefficient | Standardized coefficient | ||||
|---|---|---|---|---|---|
| Model | B | SE | β | t | P-value |
| (Constant) | 4.308 | 61.353 | 0.070 | 0.944 | |
| Maximum diameter of the tumor | 28.935 | 14.069 | 0.261 | 2.057 | 0.044 |
R=0.261, R2=0.068, RC2=0.052, F=4.230, Model utility test: P=0.044. n=60. SE, standard error.
Acknowledgements
This study was supported by a grant from the Technology Planning Project of Wenzhou Science & Technology Bureau (grant no. Y20130383).
Glossary
Abbreviations
- DST
double-stapling technique
- CT
computerized tomography
- BMI
body mass index
- TME
total mesorectal excision
- APR
abdominoperineal resection
- LAR
low anterior resection
- TNM
tumor-node-metastasis
References
- 1.Dorudi S, Steele RJ, McArdle CS. Surgery for colorectal cancer. Br Med Bull. 2002;64:101–118. doi: 10.1093/bmb/64.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery - the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 3.Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH, Kim MJ, Kim H, Shinn RK. Factors influencing pathologic results after total mesorectal excision for rectal cancer: Analysis of consecutive 100 cases. Ann Surg Oncol. 2008;15:721–728. doi: 10.1245/s10434-007-9706-z. [DOI] [PubMed] [Google Scholar]
- 4.Salerno G, Daniels IR, Brown G, Norman AR, Moran BJ, Heald RJ. Variations in pelvic dimensions do not predict the risk of circumferential resection margin (CRM) involvement in rectal cancer. World J Surg. 2007;31:1313–1320. doi: 10.1007/s00268-007-9007-5. [DOI] [PubMed] [Google Scholar]
- 5.Boyle KM, Petty D, Chalmers AG, Quirke P, Cairns A, Finan PJ, Sagar PM, Burke D. MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis. 2005;7:232–240. doi: 10.1111/j.1463-1318.2005.00819.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogiso S, Yamaguchi T, Hata H, Fukuda M, Ikai I, Yamato T, Sakai Y. Evaluation of factors affecting the difficulty of laparoscopic anterior resection for rectal cancer: Narrow pelvis is not a contraindication. Surg Endosc. 2011;25:1907–1912. doi: 10.1007/s00464-010-1485-0. [DOI] [PubMed] [Google Scholar]
- 7.Salerno G, Daniels IR, Brown G, Heald RJ, Moran BJ. Magnetic resonance imaging pelvimetry in 186 patients with rectal cancer confirms an overlap in pelvic size between males and females. Colorectal Dis. 2006;8:772–776. doi: 10.1111/j.1463-1318.2006.01090.x. [DOI] [PubMed] [Google Scholar]
- 8.Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc. 2010;24:2974–2979. doi: 10.1007/s00464-010-1075-1. [DOI] [PubMed] [Google Scholar]
- 9.Veenhof AA, Engel AF, van der Peet DL, Sietses C, Meijerink WJ, de Lange-de Klerk ES, Cuesta MA. Technical difficulty grade score for the laparoscopic approach of rectal cancer: A single institution pilot study. Int J Colorectal Dis. 2008;23:469–475. doi: 10.1007/s00384-007-0433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobin LH, Brierly J. Colon and rectum. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. 7th. Oxford: Wiley-Blackwell; 2009. pp. 100–105. [Google Scholar]
- 11.Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg. 2008;247:642–649. doi: 10.1097/SLA.0b013e3181612c6a. [DOI] [PubMed] [Google Scholar]
- 12.Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009;146:483–489. doi: 10.1016/j.surg.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Bo XF, Xiong CY, Wu AW, Zhang XP, Li M, An Q, Fang J, Li J, Zhang X, et al. Defining pelvic factors in sphincter-preservation of low rectal cancer with a three-dimensional digital model of pelvis. Dis Colon Rectum. 2006;49:1517–1526. doi: 10.1007/s10350-006-0665-4. [DOI] [PubMed] [Google Scholar]
- 14.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: A prospective evaluation of 622 patients. Ann Surg. 2004;240:260–268. doi: 10.1097/01.sla.0000133185.23514.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heald RJ, Karanjia ND. Results of radical surgery for rectal cancer. World J Surg. 1992;16:848–857. doi: 10.1007/BF02066981. [DOI] [PubMed] [Google Scholar]
- 16.Görög D, Tóth A, Péter A, Perner F. Is obesity a favorable factor for resectability of rectal cancer? Hepatogastroenterology. 2004;51:630–633. [PubMed] [Google Scholar]