Abstract
Esophageal squamous cell carcinoma (ESCC) is the eighth most frequent neoplasm in China. However, the expression levels of human epidermal growth factor receptor 2 (HER2) and multidrug resistance protein 1 (MRP1) in patients with ESCC remain to be determined. In the present study, 829 ESCC cases were evaluated using immunohistochemistry. The association between the expression levels of HER2 and MRP1 and the patient's clinicopathological factors was analyzed using Fisher's exact test or χ2 test. Univariate analysis was performed via Kaplan-Meier survival curves, while the Cox proportional hazard model was used for multivariate analysis. A significant correlation was observed between the expression levels of HER2 and the patient's gender (P<0.050), tumor size (P=0.013) and venous/lymphatic invasion (P=0.039). However, no significant correlation was identified between the expression levels of MRP1 and the clinicopathological factors of the patients. In univariate analysis, gender, differentiation, depth of invasion, clinical stage, adjuvant radiotherapy or chemotherapy and lymph node metastasis were significantly correlated with progression-free survival (PFS) and overall survival (OS) in patients with ESCC (P<0.050). The graphical representation of the Kaplan-Meier estimate curves suggested that the expression levels of HER2 or MRP1 did not exert any influence on prognosis (log-rank test, P>0.050). In multivariate analysis, tumor location, gender, clinical stage, differentiation and lymph node metastasis were identified as independent factors of prognosis in patients with ESCC (P<0.050). However, the expression levels of HER2 or MRP1 were not independently associated with PFS or OS in these patients. In conclusion, the present large-scale study demonstrates that the protein expression levels of HER2 and MRP1 does not exert any influence on the prognosis of ESCC.
Keywords: esophageal cancer, operation, HER2, MRP1, prognosis
Introduction
Esophageal carcinoma (EC) is one of the most common malignancies in the world, while esophageal squamous cell carcinoma (ESCC) is the main histopathological subtype of EC in Eastern Asian countries, including China (1). Although the therapeutic treatments for ESCC such as surgery, chemotherapy, radiotherapy and target therapy, have improved in recent years, the five-year survival rate for patients with resectable disease is <40% (2,3). Thus, an increasing interest exist on the prognostic and therapeutic value of biological markers that participate in the carcinogenesis and progression of ESCC.
Human epidermal growth factor receptor (EGFR) 2 (HER2), also known as Erb-B2 receptor tyrosine kinase 2 and c-erbB2, is a member of the EGFR family, whose abnormal activation appears to be involved in tumor development and progression in ESCC (4,5). In addition, HER2 is a therapeutic target in several types of cancer. Previous studies have observed that the oncogene HER2/neu was overexpressed in 2.0–19.1% cases of ESCC (6–13), and increased protein expression levels of HER2 have been previously observed in EC (14,15) and ESCC (6,7). However, the role of HER2 in ESCC remains controversial (6,7,9,10,12,16–19). Multidrug resistance protein 1 (MRP1), as a member of the adenosine triphosphate-binding cassette transporter family, has been implicated in resistance to cancer therapeutics (20). High expression levels of MRP1 have been observed in various solid tumors such as lung cancer, and the expression levels of MRP1 have been reported to inversely correlate with prognosis in patients with lung cancer (21–23). In addition, patients with Barrett's carcinoma treated with neoadjuvant chemotherapy who exhibited high messenger RNA expression levels of MRP1 presented prolonged survival, compared with those patients whose levels of MRP1 were low (24). However the role of MRP1 expression in patients with ESCC remains unclear.
The aim of the present retrospective study was to determine the clinical significance of HER2 and MRP1 expression in a large-scale cohort study involving patients with ESCC who had undergone surgical resection. For that purpose, the protein expression levels of HER2 and MRP1 were detected by immunohistochemistry (IHC), and the association between the clinicopathological features of this disease and the prognostic value of HER2 and MRP1 expression in patients with ESCC was evaluated.
Materials and methods
Patients
Between June 2002 and June 2010, all the consecutive cases of patients with clinical resectable ESCC who had been treated at the Department of Medical Oncology of Zhejiang Cancer Hospital (Hangzhou, China) were retrospectively reviewed. The present study was approved by the institutional review board of Zhejiang Cancer Hospital, and all the patients provided written informed consent for participation in the study. All the patients included in the study had been subjected to complete resection, and none had received neoadjuvant treatment. Those patients who succumbed to the disease within 30 days following surgery were excluded from the study. Cancer stage was determined during the postoperative pathological examination, and included the status of primary tumor invasion, regional lymph nodes and distant metastases, according to the 7th edition of the Cancer Staging Manual published by the American Joint Committee on Cancer (25). Long-term postoperative follow-up consisted of a telephone interview conducted every three months for the first three years, every six months during the fourth and fifth year, and every year thereafter. The date of the last follow-up was April 30, 2014. Overall survival (OS) was defined from the time of diagnosis until the date of mortality or until the date of the last follow-up visit. Progression-free survival (PFS) was measured from the time of completion of the surgery until the time of documented tumor recurrence or mortality. The present study was conducted according to the REporting recommendations for tumor MARKer prognostic studies guidelines (26).
Immunohistochemistry
Immunohistochemical analyses were performed by the avidin-biotin peroxidase method, using ultraView Universal DAB Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ, USA). For this purpose, paraffin sections of 4 mm thickness were excised from paraffin blocks, and subsequently immunostained with rabbit monoclonal primary antibodies against HER2 (catalogue no. 4B5; dilution, 1:100; Roche Diagnostics GmbH, Mannheim, Germany) and MRP1 (catalogue no. H-70; dilution, 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The primary antibodies were detected with an automated staining system (BenchMark XT; Roche Diagnostics GmbH).
Next, the slides were washed with phosphate-buffered saline (PBS) three times, and incubated with the secondary antibody provided in the ultraView DAB Detection Kit (Fuzhou Maixin Biotech. Co., Ltd, Fujian, China). The colorimetric reaction was developed with 3,3′-diaminobenzidine. Counterstaining was performed with hematoxylin. All steps were conducted at room temperature. For the negative control, the primary antibody was replaced with PBS.
The immunostaining results were independently examined by two clinical pathologists who were blinded to the patients' information, using a DM4000B light microscope (Leica, Wetzlar, Germany). For each sample, five high-power fields (magnification, ×400) were randomly selected. The percentage of positive cells and the intensity of the staining were assessed, and a semi-quantitatively score ranging from 0 to 3 was assigned to the samples accordingly. The staining intensity was scored as follows: i) 0, no staining; ii) 1, weak staining; iii) 2, moderate staining; and iv) 3, strong staining. This score was then multiplied by the percentage of positively stained cells present in the sample, as follows: i) 0, no staining; ii) 1, ≤10% of stained cells; iii) 2, 11–25% of stained cells; iv) 3, 26–50% of stained cells; and v) 4, ≥51% of stained cells. The resulting scores were used to classify the samples as follows: i) 0, negative staining; ii) 1–4, weak positive staining; iii) 5–8, moderate staining; and iv) 9–12, strong positive staining.
Statistical analysis
The correlation between the expression levels of HER2 and MRP1 and the patient's clinicopathological factors was analyzed using Fisher's exact test or χ2 test. Univariate and multivariate analyses were performed with Kaplan-Meier survival curves and Cox proportional hazard model, respectively. Log-rank test was used to evaluate the significance of the differences observed between pairs of survival probabilities. P<0.050 was considered to indicate a statistically significant difference. Statistical data were calculated with SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient's characteristics
The cohort of 829 patients with ESCC whose expression levels of MRP1 were analyzed in the present study exhibited a female:male ratio of 1.0:4.5 and a median age of 59 years (range, 34–78 years). Among the 829 patients, 768 patients also detected HER-2 expression. ESCC was observed to be well differentiated in 110 cases (13.3%), moderately differentiated in 561 cases (67.7%), poorly differentiated in 153 cases (18.4%) and undifferentiated in 5 cases (0.6%). The enrolled patients were staged pathologically as I (n=61), II (n=335) and III (n=433). Complete resection was confirmed histologically in all patients. Locoregional lymph node metastasis was observed in 477 patients (57.5%). A total of 260 patients who presented poor prognostic factors, metastatic disease or recurrence following operation, received adjuvant radiotherapy and/or chemotherapy subsequently to surgery. A total of 762 patients were available for survival analysis, with a median duration of postoperative follow-up of 32 months (range, 1.03–102.00 months). Among them, 733 patients were followed-up for >3 years. The clinicopathological factors, staging and survival of the patients included in the present study have been previously reported (27,28).
Association between HER2 and MRP1 expression and clinicopathological features
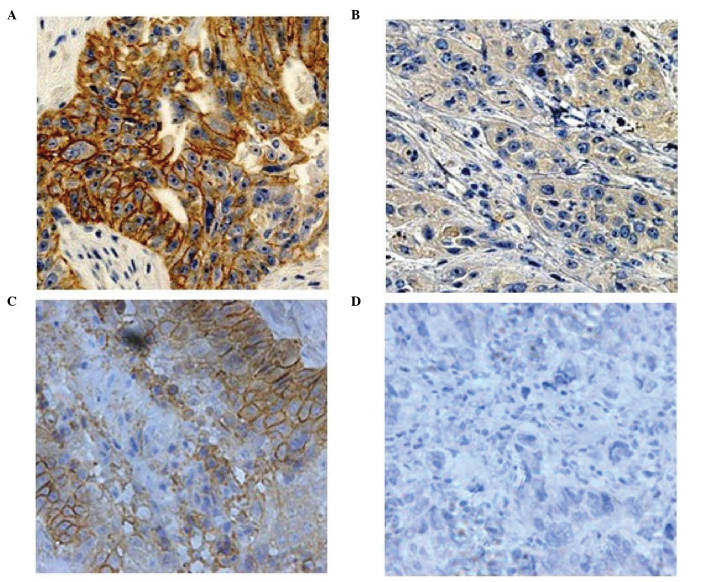
In ESCC tissues, HER2 exhibited membrane staining, while MRP1 displayed membrane and cytoplasmic staining (Fig. 1). A total of 768 patients were available for evaluation of HER2 expression. Among them, 543 cases (70.7%) were observed to be negative, 170 cases (22.1%) were scored as 1, 37 cases (4.8%) as 2 and 18 cases (2.3%) as 3. For statistical analysis, samples with a final score of 0 were considered to exhibit low expression levels of HER2, whereas those with a final score of 1–3 were considered to display high expression levels of HER2. Among the 829 cases who were assessed for MRP1 expression by IHC, 326 (39.3%), 291 (35.1%), 144 (17.4%) and 68 (8.2%) cases were scored as 0, 1, 2 and 3, respectively. For statistical analysis, samples with a final score of 0 or 1 were considered to exhibit low expression levels of MRP1, while those with a final score of 2 or 3 were considered to display high expression levels of MRP1.
Figure 1.
Immunohistochemical staining of HER2 and MRP1 in esophageal squamous cell carcinoma tissue. (A and C) Positive and (B and D) negative protein expression of HER2 and MRP1, respectively (magnification, ×400). HER2, human epidermal growth factor receptor 2; MRP1, multidrug resistance protein 1.
The associations between the expression levels of HER2 and MRP1 and the clinicopathological features of the patients are presented in Table I. A significant correlation was observed between the expression levels of HER2 and gender (P<0.050), tumor size (P=0.013) and venous/lymphatic invasion (P=0.039). However, no significant correlation was identified between MRP1 expression and any of the clinicopathological factors analyzed. Furthermore, high expression levels of HER2 were positively correlated with high expression levels of MRP1 (P=0.001).
Table I.
Analysis of the association between the expression levels of HER2 and MRP1 and the clinicopathological features of patients with esophageal squamous cell carcinoma.
| HER2 expression levels (no. of patients) | MRP1 expression levels (no. of patients) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Total | Low | High | P-value | Total | Low | High | P-value |
| Gender | 0.000 | 0.516 | ||||||
| Male | 627 | 466 | 161 | 678 | 499 | 179 | ||
| Female | 141 | 77 | 64 | 151 | 115 | 36 | ||
| Age (years) | 0.760 | 0.702 | ||||||
| ≥55 | 491 | 349 | 142 | 527 | 388 | 139 | ||
| <55 | 277 | 194 | 83 | 302 | 226 | 76 | ||
| Smoking | 0.163 | 0.532 | ||||||
| Never | 179 | 134 | 45 | 202 | 153 | 49 | ||
| Ever | 589 | 409 | 180 | 627 | 461 | 166 | ||
| Alcohol consumption | 0.178 | 0.755 | ||||||
| Never | 256 | 189 | 67 | 282 | 207 | 75 | ||
| Ever | 512 | 354 | 158 | 547 | 407 | 140 | ||
| Differentiation | 0.216 | 0.055 | ||||||
| Intermediate/well | 622 | 447 | 175 | 671 | 507 | 164 | ||
| Poor/undifferentiated | 144 | 96 | 48 | 158 | 107 | 51 | ||
| Tumor size (cm) | 0.013 | 0.671 | ||||||
| <5 | 442 | 297 | 145 | 464 | 341 | 123 | ||
| ≥5 | 326 | 246 | 80 | 365 | 273 | 92 | ||
| Depth of invasion | 0.678 | 0.362 | ||||||
| T1+T2 | 160 | 111 | 49 | 171 | 122 | 49 | ||
| T3 | 608 | 432 | 176 | 658 | 492 | 166 | ||
| Lymph node metastasis | 0.347 | 0.126 | ||||||
| N0 | 321 | 232 | 89 | 352 | 273 | 79 | ||
| N1 | 214 | 148 | 66 | 232 | 162 | 70 | ||
| N2 | 159 | 106 | 53 | 169 | 120 | 49 | ||
| N3 | 74 | 57 | 17 | 76 | 59 | 17 | ||
| Clinical stage | 0.647 | 0.456 | ||||||
| I+II | 358 | 256 | 102 | 396 | 298 | 98 | ||
| III | 410 | 287 | 123 | 433 | 316 | 117 | ||
| Venous/lymphatic invasion | 0.039 | 0.094 | ||||||
| No | 622 | 450 | 172 | 672 | 506 | 166 | ||
| Yes | 146 | 93 | 53 | 157 | 108 | 49 | ||
| Perineural invasion | 0.050 | 0.219 | ||||||
| No | 588 | 426 | 162 | 638 | 466 | 172 | ||
| Yes | 180 | 117 | 63 | 191 | 148 | 43 | ||
| Adjuvant radio/chemotherapy | 0.269 | 0.196 | ||||||
| No | 517 | 359 | 158 | 569 | 429 | 140 | ||
| Yes | 251 | 184 | 67 | 260 | 185 | 75 | ||
HER2, human epidermal growth factor receptor 2; MRP1, multidrug resistance protein 1.
Survival analysis
During the follow-up, 397 patients succumbed to ESCC, while recurrence or metastasis occurred in 470 patients. The metastatic areas included the supraclavicular lymph node, mediastinal lymph node, liver, lung, skeleton and brain.
In univariate analysis, gender, differentiation, depth of invasion, clinical stage, adjuvant radiotherapy or chemotherapy and lymph node metastasis were significantly correlated with the patients' PFS and OS (P<0.050) (Table II). However, the graphic pattern of the Kaplan-Meier estimate curves (Fig. 2) suggested that the expression levels of HER2 or MRP1 did not have any impact on prognosis (log-rank, P>0.050). In multivariate analysis, tumor location, clinical stage and lymph node metastasis were identified as independent factors of prognosis in patients with ESCC (Table III). However, the expression levels of HER2 or MRP1 were not observed to be independently associated with PFS and OS in these patients (Table III).
Table II.
Univariate analysis of factors that influence the survival of patients with esophageal squamous cell carcinoma.
| Progression-free survival | Overall survival | |||
|---|---|---|---|---|
| Factors | No. of patients | P-value | No. of patients | P-value |
| Gender | 0.009 | 0.018 | ||
| Male | 554 | 645 | ||
| Female | 108 | 129 | ||
| Age (years) | 0.339 | 0.843 | ||
| ≥55 | 416 | 491 | ||
| <55 | 246 | 283 | ||
| Smoking | 0.315 | 0.177 | ||
| Never | 156 | 186 | ||
| Ever | 506 | 588 | ||
| Alcohol consumption | 0.869 | 0.199 | ||
| Never | 218 | 266 | ||
| Ever | 444 | 508 | ||
| Location | 0.400 | 0.149 | ||
| Upper/middle | 274 | 338 | ||
| Lower | 388 | 436 | ||
| Differentiation | 0.000 | 0.000 | ||
| Intermediate/well | 528 | 618 | ||
| Poor/undifferentiated | 134 | 155 | ||
| Tumor size (cm) | 0.164 | 0.023 | ||
| <5 | 369 | 444 | ||
| ≥5 | 293 | 330 | ||
| Depth of invasion | 0.000 | 0.000 | ||
| T1+T2 | 131 | 168 | ||
| T3 | 531 | 606 | ||
| Lymph node metastasis | 0.000 | 0.000 | ||
| N0 | 261 | 331 | ||
| N1 | 178 | 212 | ||
| N2 | 152 | 160 | ||
| N3 | 71 | 71 | ||
| Clinical stage | 0.000 | 0.000 | ||
| I+II | 297 | 374 | ||
| III | 365 | 400 | ||
| Venous/lymphatic invasion | 0.473 | 0.373 | ||
| No | 531 | 629 | ||
| Yes | 131 | 145 | ||
| Perineural invasion | 0.203 | 0.919 | ||
| No | 531 | 604 | ||
| Yes | 131 | 170 | ||
| Adjuvant radio/chemotherapy | 0.049 | 0.002 | ||
| No | 425 | 524 | ||
| Yes | 237 | 250 | ||
| Human epidermal growth factor receptor 2 | 0.373 | 0.196 | ||
| Low expression levels | 432 | 515 | ||
| High expression levels | 178 | 194 | ||
| Multidrug resistance protein 1 | 0.338 | 0.661 | ||
| Low expression levels | 423 | 496 | ||
| High expression levels | 239 | 278 | ||
Figure 2.
Kaplan-Meier estimate curves of (A and C) progression-free survival and (B and D) overall survival in patients with esophageal squamous cell carcinoma, according to the protein expression levels of HER2 and MRP1, respectively. HER2, human epidermal growth factor receptor 2; MRP1, multidrug resistance protein 1.
Table III.
Multivariate analysis of progression-free survival and overall survival in patients with esophageal squamous cell carcinoma.
| Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Factors | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Gender | 0.021 | 0.051 | ||||
| Male | Ref. | Ref. | ||||
| Female | 0.732 | 0.563–0.954 | 0.756 | 0.567–1.008 | ||
| Tumor location | 0.027 | NR | ||||
| Upper/middle | Ref. | NR | ||||
| Lower | 1.235 | 1.024–1.489 | NR | NR | ||
| Clinical stage | 0.020 | 0.014 | ||||
| I+II | Ref. | Ref. | ||||
| III | 1.507 | 1.069–2.129 | 1.640 | 1.104–2.435 | ||
| Lymph node metastasis | 0.000 | 0.000 | ||||
| N0 | Ref. | Ref. | ||||
| N1 | 1.170 | 0.826–1.656 | 1.342 | 0.893–2.015 | ||
| N2 | 1.516 | 1.021–2.251 | 2.181 | 1.395–3.409 | ||
| N3 | 2.260 | 1.463–3.491 | 1.640 | 1.104–2.435 | ||
| Differentiation | 0.061 | 0.085 | ||||
| Intermediate/well | Ref. | Ref. | ||||
| Poor/undifferentiated | 1.240 | 0.999–1.553 | 1.233 | 0.972–1.564 | ||
CI, confidence interval; Ref., reference; NR, not reported.
Discussion
HER2 is a therapeutic target in several tumors such as breast cancer (29–32), and a predictive factor of response to chemotherapy in various solid malignancies (33–36). In order to identify improved prognostic indicators for ESCC, the clinicopathological features of patients with ESCC were investigated in the present study. In previous studies, the number of copies of the HER2/neu gene was analyzed by fluorescence or chromogenic in situ hybridization, and the protein expression levels of HER2 were evaluated by IHC (6,7,9,10,12,16–19). Previous studies examining the association between the expression levels of HER2 (as determined by IHC) and prognosis in patients with ESCC have reported conflicting results (Table IV). These discrepancies may be due to the following reasons: i) Different quality of the studies; ii) multiple factors that may influence prognosis were not considered in all the studies; iii) different laboratory techniques were employed in different studies, including the use of different antibodies to detect HER2 and MRP1; and iv) small size of the samples used in the studies (≤250 patients; Table IV). The association between HER2 expression and prognosis in EC has not been reported thus far.
Table IV.
Previous studies describing the prognostic significance of the expression levels of human epidermal growth factor receptor 2 in ESCC, as determined by IHC or ISH.
| First author, year (reference) | Type of cancer | Prognostic effect | Sample size (no. of patients) | Method |
|---|---|---|---|---|
| Hardwick, 1997 (19) | ESCC, AC | No effect | 205 | IHC |
| Friess, 1999 (12) | ESCC, AC | No effect | 39 | IHC |
| Mimura, 2005 (9) | ESCC | Unfavorable | 66 | IHC, ISH |
| Reichelt, 2007 (10) | ESCC, AC | No effect | 251 | IHC, ISH |
| Sato-Kuwabara, 2009 (6) | ESCC | Unfavorable | 188 | IHC, ISH |
| Stoecklein, 2008 (37) | ESCC, AC | No effect | 101 | ISH |
| Schoppmann, 2010 (11) | ESCC, AC | No effect | 341 | IHC, ISH |
| Birner, 2011 (38) | ESCC, AC | Unfavorable | 330 | IHC, ISH |
| Zhan, 2012 (7) | ESCC | Unfavorable | 145 | IHC, ISH |
| Kato, 2013 (16) | ESCC | No effect | 245 | IHC, ISH |
| Wang, 2013 (39) | ESCC | Unfavorable | 72 | IHC |
| Wang, 1999 (18) | ESCC | No effect | 117 | IHC |
ESCC, esophageal squamous cell carcinoma; AC, adenocarcinoma; IHC, immunohistochemistry; ISH, in situ hybridization.
In the present study, a large cohort of 829 patients with ESCC and long follow-up was analyzed, and no correlation was observed between the expression levels of HER2 or MRP1 and the postoperative survival time exhibited by these patients. To the best of our knowledge, the present study is the first large-scale study conducted to evaluate the prognostic role of MRP1 expression in patients with ESCC who had been treated with primary surgery. In the present study, a significant association was observed between survival and clinical stage, lymph node metastasis and poor differentiation in patients with ESCC. However, the present study is affected by certain limitations, including its retrospective and single-hospital nature. Thus, multicenter and prospective studies are required to further validate the results of the present study. In addition, the protein expression levels of HER2 and MRP1 were solely examined by IHC in the present study, which may not be completely consistent with their gene expression levels.
In summary, the results of the present study indicated that the expression levels of MRP1 and HER2, as determined by IHC analysis, were not associated with survival rate in patients with ESCC. This result indicates that HER2 or MRP1 expression may not serve as informative prognostic biomarkers. Therefore, further studies are required to clarify the mechanism behind the expression of MRP1 and HER2 in patients with ESCC.
Acknowledgements
The present study was supported by the Province Important Technology and Science Program (Special Feature of Major Province Scientific and Technological Program 2011, grant no. 2011C13039-1), and the National Natural Science Foundation of China (Beijing, China) (General Program; grant nos. 81172081 and 81372210).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. MAGIC Trial Participants: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, Ten Kate FJ, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: Five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1001. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 4.Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK. Signal events: Cell signal transduction and its inhibition in cancer. Oncologist. 2003;8(Suppl 3):5–17. doi: 10.1634/theoncologist.8-suppl_3-5. [DOI] [PubMed] [Google Scholar]
- 6.Sato-Kuwabara Y, Neves JI, Fregnani JH, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER2/neu in esophageal squamous cell carcinoma using Fluorescence in situ Hybridization (FISH) and immunohistochemistry. BMC Cancer. 2009;9:6. doi: 10.1186/1471-2407-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan N, Dong WG, Tang YF, Wang ZS, Xiong CL. Analysis of HER2 gene amplification and protein expression in esophageal squamous cell carcinoma. Med Oncol. 2012;29:933–940. doi: 10.1007/s12032-011-9850-y. [DOI] [PubMed] [Google Scholar]
- 8.Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal cancer. J Gastrointest Surg. 2012;16:1821–1829. doi: 10.1007/s11605-012-1979-2. [DOI] [PubMed] [Google Scholar]
- 9.Mimura K, Kono K, Hanawa M, Mitsui F, Sugai H, Miyagawa N, Ooi A, Fujii H. Frequencies of HER2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2005;92:1253–1260. doi: 10.1038/sj.bjc.6602499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichelt U, Duesedau P, Tsourlakis MCh, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A, et al. Frequent homogeneous HER2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120–129. doi: 10.1038/modpathol.3800712. [DOI] [PubMed] [Google Scholar]
- 11.Schoppmann SF, Jesch B, Friedrich J, Wrba F, Schultheis A, Pluschnig U, Maresch J, Zacherl J, Hejna M, Birner P. Expression of HER2 in carcinomas of the esophagus. Am J Surg Pathol. 2010;34:1868–1873. doi: 10.1097/PAS.0b013e3181f8be17. [DOI] [PubMed] [Google Scholar]
- 12.Friess H, Fukuda A, Tang WH, Eichenberger A, Furlan N, Zimmermann A, Korc M, Büchler MW. Concomitant analysis of the epidermal growth factor receptor family in esophageal cancer: Overexpression of epidermal growth factor receptor mRNA but not of c-erbB-2 and c-erbB-3. World J Surg. 1999;23:1010–1018. doi: 10.1007/s002689900616. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Abo S, Kitamura M, Hashimoto M, Izumi K, Terada K, Sugiyama T. Gene amplification of int-2 and erbB in human esophageal cancer: Relationship to clinicopathological variables. Cancer Invest. 1997;15:411–415. doi: 10.3109/07357909709047579. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Nekarda H, Hoelscher AH, Bollschweiler E, Harbeck N, Becker K, Siewert JR, Harbec N. Prognostic value of DNA ploidy and c-erbB-2 oncoprotein overexpression in adenocarcinoma of Barrett's esophagus. Cancer. 1994;73:1785–1794. doi: 10.1002/1097-0142(19940401)73:7<1785::AID-CNCR2820730703>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Polkowski W, van Sandick JW, Offerhaus GJ, ten Kate FJ, Mulder J, Obertop H, van Lanschot JJ. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290–297. doi: 10.1007/s10434-999-0290-2. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Arao T, Matsumoto K, Fujita Y, et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol. 2013;42:1151–1158. doi: 10.3892/ijo.2013.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson SK, Sullivan TR, Davies R, Ruszkiewicz AR. HER2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Ann Surg Oncol. 2011;18:2010–2017. doi: 10.1245/s10434-011-1554-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang LS, Chow KC, Chi KH, Liu CC, Li WY, Chiu JH, Huang MH. Prognosis of esophageal squamous cell carcinoma: Analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94:1933–1940. doi: 10.1111/j.1572-0241.1999.01233.x. [DOI] [PubMed] [Google Scholar]
- 19.Hardwick RH, Barham CP, Ozua P, Newcomb PV, Savage P, Powell R, Rahamin J, Alderson D. Immunohistochemical detection of p53 and c-erbB-2 in oesophageal carcinoma; no correlation with prognosis. Eur J Surg Oncol. 1997;23:30–35. doi: 10.1016/S0748-7983(97)80139-4. [DOI] [PubMed] [Google Scholar]
- 20.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 21.Galimberti S, Marchetti A, Buttitta F, Carnicelli V, Pellegrini S, Bevilacqua G, Petrini M. Multidrug resistance related genes and p53 expression in human non small cell lung cancer. Anticancer Res. 1998;18:2973–2976. [PubMed] [Google Scholar]
- 22.Yokoyama H, Ishida T, Sugio K, Inoue T, Sugimachi K. Immunohistochemical evidence that P-glycoprotein in non-small cell lung cancers is associated with shorter survival. Surg Today. 1999;29:1141–1147. doi: 10.1007/BF02482262. [DOI] [PubMed] [Google Scholar]
- 23.Tews DS, Nissen A, Külgen C, Gaumann AK. Drug resistance-associated factors in primary and secondary glioblastomas and their precursor tumors. J Neurooncol. 2000;50:227–237. doi: 10.1023/A:1006491405010. [DOI] [PubMed] [Google Scholar]
- 24.Langer R, Specht K, Becker K, Ewald P, Bekesch M, Sarbia M, Busch R, Feith M, Stein HJ, Siewert JR, Höfler H. Association of pretherapeutic expression of chemotherapy-related genes with response to neoadjuvant chemotherapy in Barrett carcinoma. Clin Cancer Res. 2005;11:7462–7469. doi: 10.1158/1078-0432.CCR-05-0042. [DOI] [PubMed] [Google Scholar]
- 25.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th. Hoboken, NJ: Wiley-Blackwell; 2009. Title section/chapter; p. 455. [Google Scholar]
- 26.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics: REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu XL, Zheng WH, Fu ZX, Li ZP, Xie HX, Li XX, Jiang LH, Wang Y, Zhu SM, Mao WM. Topo2A as a prognostic biomarker for patients with resectable esophageal squamous cell carcinomas. Med Oncol. 2015;32:396. doi: 10.1007/s12032-014-0396-7. [DOI] [PubMed] [Google Scholar]
- 28.Xu XL, Zheng WH, Tao KY, Li XX, Xu WZ, Wang Y, Zhu SM, Mao WM. p53 is an independent prognostic factor in operable esophageal squamous cell carcinoma: A large-scale study with a long follow-up. Med Oncol. 2014;31:257. doi: 10.1007/s12032-014-0257-4. [DOI] [PubMed] [Google Scholar]
- 29.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, et al. Quantitative association between HER2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 30.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6,556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/CBC.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 31.Parums DV. Current status of targeted therapy in non-small cell lung cancer. Drugs Today (Barc) 2014;50:503–525. doi: 10.1358/dot.2014.50.07.2185913. [DOI] [PubMed] [Google Scholar]
- 32.Pollock NI, Grandis JR. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:526–533. doi: 10.1158/1078-0432.CCR-14-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kontopodis E, Kentepozidis N, Christophyllakis CH, Boukovinas I, Kalykaki A, Kalbakis K, Vamvakas L, Agelaki S, Kotsakis A, Vardakis N, et al. Docetaxel, gemcitabine and bevacizumab as salvage chemotherapy for HER2-negative metastatic breast cancer. Cancer Chemother Pharmacol. 2015;75:153–160. doi: 10.1007/s00280-014-2628-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee HJ, Seo AN, Kim EJ, Jang MH, Suh KJ, Ryu HS, Kim YJ, Kim JH, Im SA, Gong G, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014;142:755–766. doi: 10.1309/AJCPIRL4GUVGK3YX. [DOI] [PubMed] [Google Scholar]
- 35.Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, Gralow J, Hortobagyi GN, Moy B, Yee D, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32:3307–3329. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W, Barón AE, Zeng C, Johnson TK, Bunn PA., Jr The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11:795–805. [PubMed] [Google Scholar]
- 37.Stoecklein NH, Hosch SB, Bezler M, Stern F, Hartmann CH, Vay C, Siegmund A, Scheunemann P, Schurr P, Knoefel WT, et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell. 2008;13:441–453. doi: 10.1016/j.ccr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Birner P, Jesch B, Friedrich J, Riegler M, Zacherl J, Hejna M, Wrba F, Schultheis A, Schoppmann SF. Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with HER2 expression. Ann Surg Oncol. 2011;18:3330–3337. doi: 10.1245/s10434-011-1730-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Zhang W, Jiang W, Luan L. Overexpression of HER2 associated with the progression of esophageal cancer patients. Hepatogastroenterology. 2013;60:1972–1978. [PubMed] [Google Scholar]