Fig. 2.
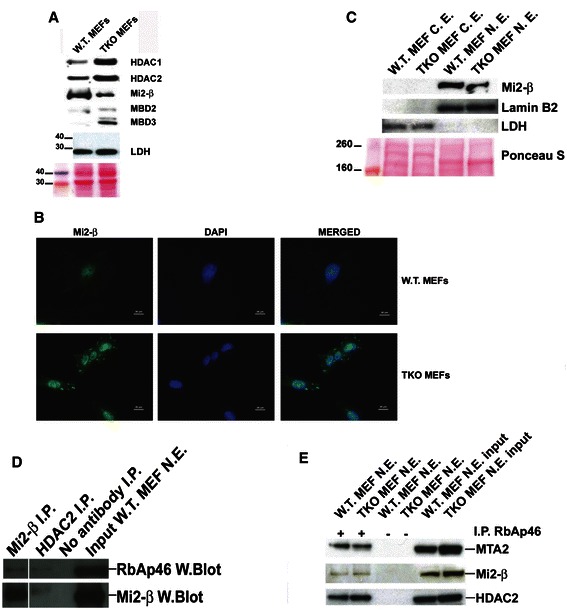
Recruitment of the NuRD corepressor complex in MEFs is influenced by RB proteins. a Wild type and TKO MEFs were grown to 90 % confluence, trypsinized and lysed using RIPA buffer supplemented with protease inhibitors. 20 μg of total protein was separated on a 4-12 % gradient PAGE gel and probed with antisera against selected targets. HRP-linked secondary antibodies were used for protein detection. Film exposure times varied from 30 s to 3 min. The abundance of Mi2-β was dramatically reduced in MEFs lacking RB proteins, while other members of the NuRD corepressor complex including, HDAC1, HDAC2 and MBD3, were increased. No differences in LDH levels and Ponceau staining as loading controls were observed. These results are representative of three independent experiments. b Immunofluorescence staining for Mi2-β and DAPI in WT and TKO MEFs. The signal in WT MEFs was confined to the nucleus, while that in TKO MEFs was distributed throughout the cell thus giving rise to lighter fluorescence signals. c Measurement of Mi2-b levels by Western blot analysis in nuclear extracts (NE) and cytosolic extracts (CE) of wild type and TKO MEFs. Lamin B2 and LDH were used as markers of purity for the nuclear and cytosolic extracts, respectively. Ponceau staining was used as a loading control for each of the fractions. While decreased nuclear Mi2-β levels as evidenced by Western blot analysis of nuclear protein in TKO cells compared to wildtype counterparts was observed, no signal was detected in the cytoplasmic fraction. d Immunoprecipitation of RbAp46 using anti-Mi2-β antibody in NE of WT MEFs. e Immunoprecipitation of MTA2, Mi2-β, and HDAC2 using anti-RbAp46 antibody in NE of WT and TKO MEFs
