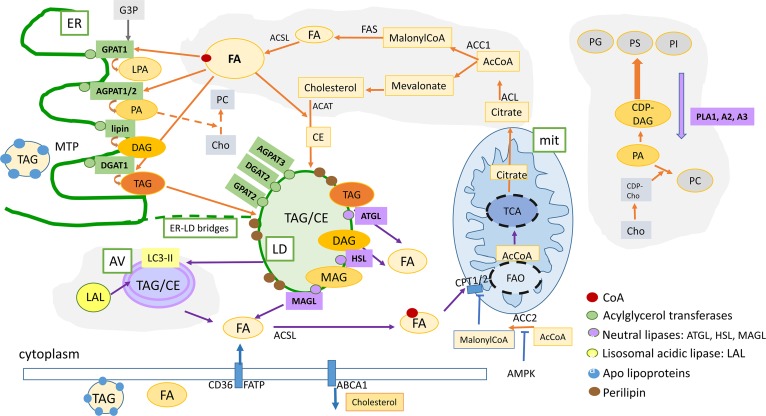
Fig. 2.
Summary of the lipid metabolism pathways that are described in this review: Lipogenesis and FAO can cooperate as partners to increase plasticity of cancer cells. De novo FA and cholesterol biosynthetic pathways and LD formation are indicated by the orange arrows. FAO contributes to increase survival under energy and oxidative stress (indicated by blue arrows). Intracellular lipid content is the result of a dynamic balance between neutral lipid storage in LDs (TAG and CE) and lipid mobilization by neutral lipases (lipolysis) or acidic lipases (lipophagy) that release FFA for FAO in mitochondria or substrates for lipid-related signaling molecules, as well as structural molecules for membrane biosynthesis. In addition, LDs contain an enriched proteome that regulates lipid mobilization and protects from toxic unfolded proteins, buffers excess of proteins, and regulates lipid mobilization when required. Lipophagy is an active process for lipid mobilization that contributes to increase the survival and fitness of cancer cells. Orange arrows, FA and lipid biosynthesis; blue arrows, lipid mobilization and FAO; green boxes, enzymes implicated in lipid biosynthesis; purple boxes, enzymes implicated in lipid mobilization [lipases (adipose TAG lipase, HSL, MAGL) and phospholipases (PLA1, PLA2, PLA3) are indicated in purple]. Lipophagy-related acidic lipases are indicated in yellow. mit, mitochondria; G3P, glycerol-3-phosphate; MAG, monoacylglycerol; CE, cholesterol esters.; PG, phosphatidylglycerol; PS, phosphatidylserine; PC, phosphatidylcholine, PE, phosphatidylethanolamine; PI, phosphatidylinositol; Cho, choline; CPT1A2, carnitine palmitoyltransferase 1A2; CD36, thrombospondin receptor; FATP, fatty acid transport protein.