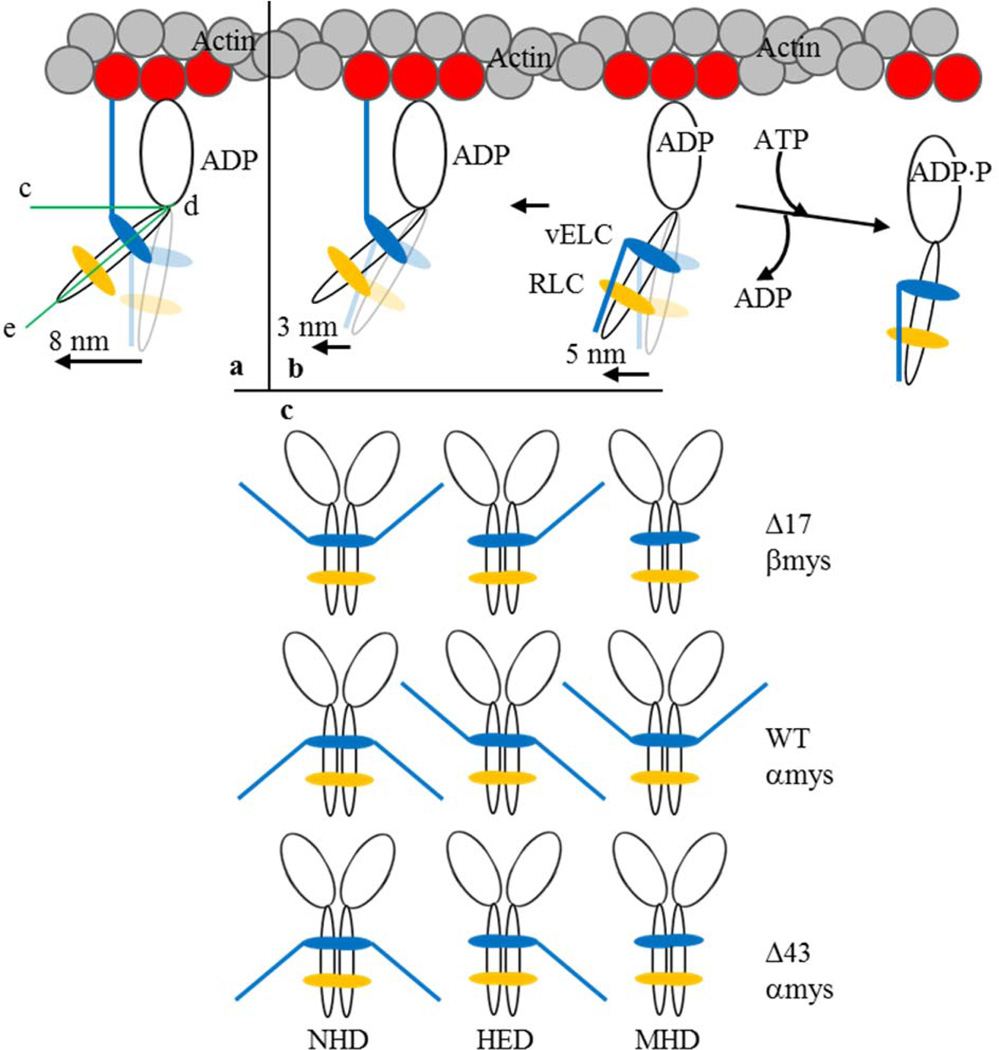
Figure 2.
The proposed 3 step-size mechanism for cardiac myosin moving actin in vitro and the myosin dimers participating in motility. Panel a is the full lever-arm swing step of 8 nm with actin binding of the vELC N-terminus. Panel b shows the 5 nm step followed by detachment from actin (towards right) or an unlikely event when lingering ADP causes a subsequent 3 nm step (towards left). The full 8 nm step facilitated by the vELC/actin link corresponds to a lever-arm rotation ~19° larger than that for a 5 nm step. Angle, ∠cde in panel a, indicates the acute angle specifying lever-arm orientation relative to the actin filament. Panel c shows the myosin dimers participating in motility and notation representing the native homodimers (NHD) containing intact vELC, the heterodimer (HED) with one modified and one native vELC, and the modified homodimer (MHD) with two modified vELC’s. Mouse vELC is distinguished from human vELC by the tip-down (mouse) or tip-up (human) ELC N-terminus.