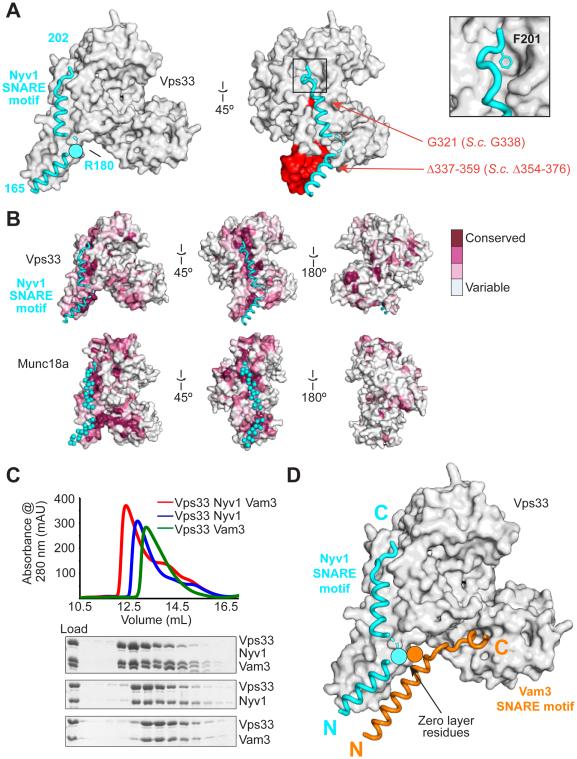
Fig. 2. Binding to a conserved surface groove on Vps33 orients and aligns the R-SNARE Nyv1 for assembly.
(A) Vps33–Nyv1 crystal structure (Vps16 omitted for clarity; see fig. S3). Red indicates mutations employed in Fig. 3. (B) The Nyv1 binding site is conserved (as calculated by ConSurf (30)) among Vps33 homologs. The same site, indicated with cyan spheres, is conserved among Munc18 homologs (shown is 3PUJ). (C) Vps33, Nyv1, and Vam3 form a ternary complex as judged by size exclusion chromatography. Nyv1 and Vam3 SNARE motifs contain an N-terminal maltose binding protein tag. (D) Model showing both SNARE domains binding simultaneously to Vps33.