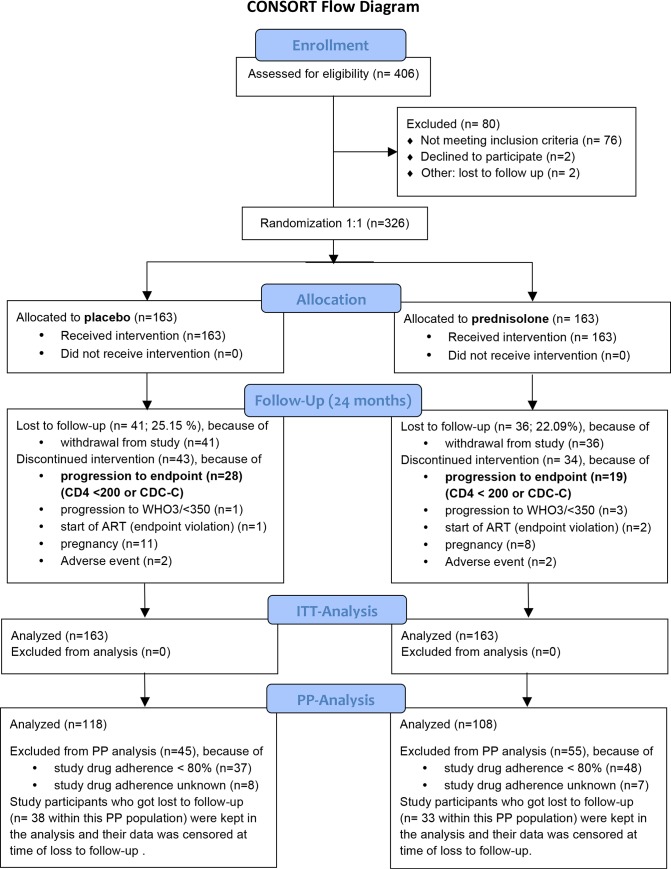
Fig 1. CONSORT statement 2010 flow diagram.
The number of participants enrolled, randomized, allocated to study medication, followed-up and analyzed is shown. Study participants who progressed to the endpoint of the study (CD4 < 200 or CDC stage-C disease) received HAART. In some cases, study participants also received HAART when they progressed to CD4 < 350 in combination with WHO stage 3-disease. This was in accordance to the National Tanzanian treatment recommendations Update in 2008 (1 case in the placebo arm and 3 cases in the prednisolone arm). In addition, one patient in the placebo arm and 2 study participants in the prednisolone arm received HAART without fulfilling either the study endpoint or the criteria listed in the National Treatment recommendation update.