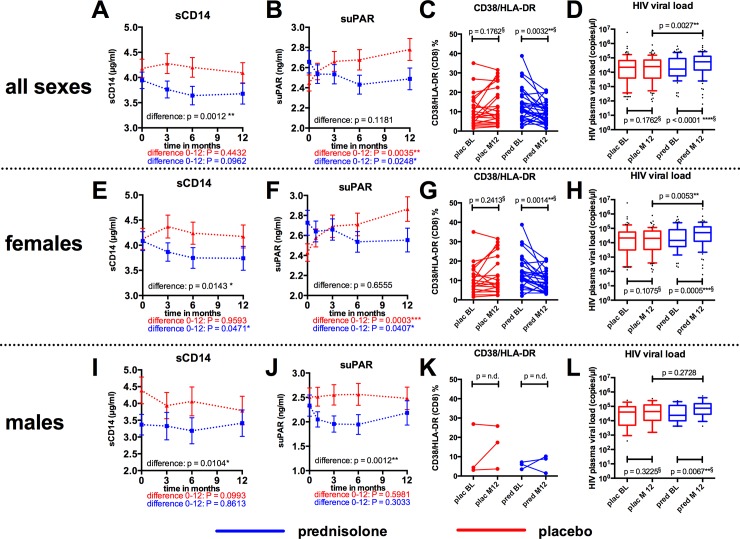
Fig 9. Effects of prednisolone on immune activation and HIV viral load.
A, E, I: Concentration of sCD14 was determined by ELISA from n = 134 (placebo) and n = 136 (prednisolone) available plasma samples collected at baseline, 3, 6 and 12 months. A: all sexes, E: females (nPlac = 106, nPred = 110), I: males (nPlac = 28, nPred = 26). B, F, J: Concentration of sUPAR was determined by ELISA from n = 122 (placebo) and n = 124 (prednisolone) available plasma samples collected at baseline, 3, 6 and 12 months. B: all sexes, F: females (nPlac = 95, nPred = 102), J: males (nPlac = 27, nPred = 22). A, B, E, F, I, J: Data as means ± S.D. P-values were determined by 2-way ANOVA (difference between the two treatments over the whole time period) or by Wilcoxon matched-pairs signed test (changes between Baseline and month 12). C, G, K: CD38/HLA-DR expression was determined by flow cytometry from n = 22 (placebo) and n = 30 (prednisolone) available frozen PBMC samples collected at baseline and 12 months. C: all sexes, G: females (nPlac = 19, nPred = 27), K: males (nPlac = 3, nPred = 3). P-values were determined by Wilcoxon matched-pairs signed test (changes between Baseline and month 12). D, H, L: HIV viral load was determined from n = 86 (placebo) and n = 80 (prednisolone) available plasma pairs at baseline and month 12. D: all sexes, H: females (nPlac = 70, nPred = 66), L: males (nPlac = 16, nPred = 14). P-values were determined by Wilcoxon matched-pairs signed test (changes between Baseline and month 12) and by Mann-Whitney test (comparison of month 12 placebo versus month 12 prednisolone).