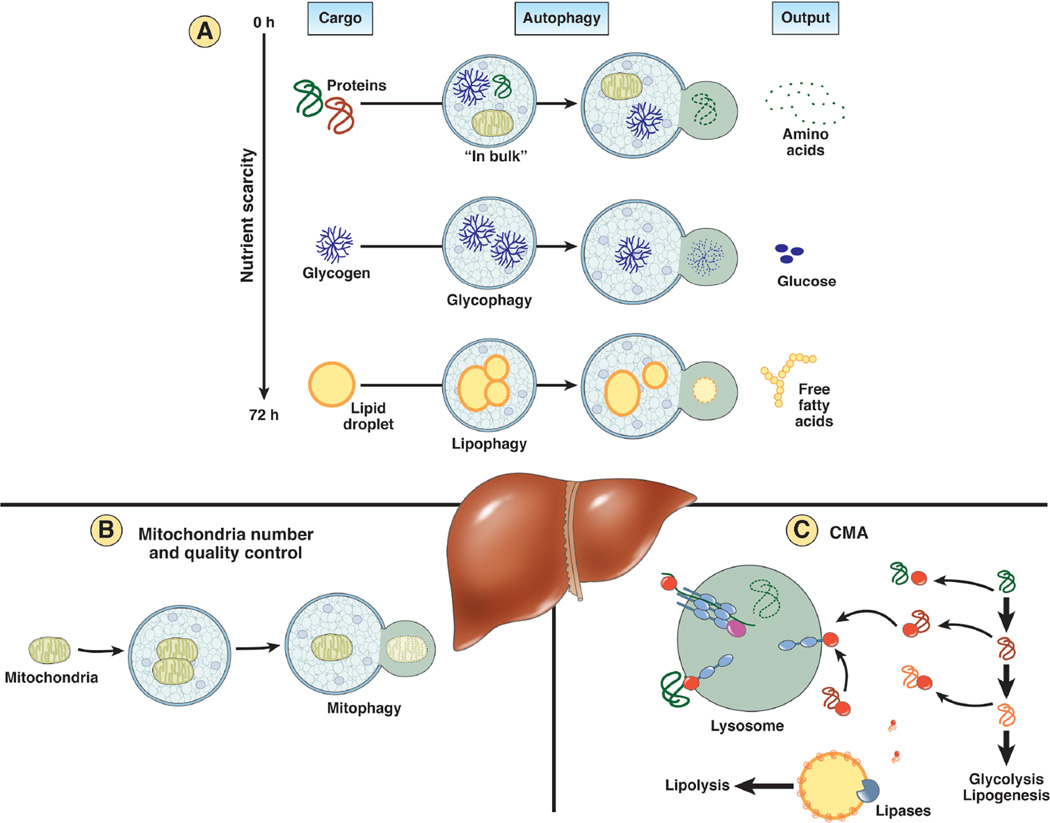
Figure 2. Three main functions of hepatic autophagy in the control of the energetic balance.
A: Autophagy recycles essential components through degradation of cellular proteins and energy stores. The type of cargo selected by autophagy, at least in liver, changes depending on the duration of nutrient scarcity. While in bulk autophagy of cytosolic proteins and organelles is predominant early in starvation and constitutes an important source of amino acids, if nutrients shortage persists, there is a switch toward glycogen and lipid droplets as preferential cargos. Glycophagy and lipohagy contribute thus glucose and free fatty acids that can be utilized to sustain a positive energetic balance in absence of nutrients. B: Autophagy also manages the cellular energetic balance through the fine-tuned regulation of mitocondrial number and quality control. Mitophagy can eliminate non-functional mitochondria but also controls mitochondrial mass through a coordinated balance with mitochondrial biogenesis. C: Autophagy contributes to accommodation to starvation and other nutritional challenges through selective removal via chaperone-mediated autophagy (CMA) of enzymes that control metabolic pathways such as glycolysis or lipogenesis. In addition, CMA also regulates hepatic lipolytic capacity through degradation of perilipins in the surface of lipid droplets. Removal of these proteins is necessary for cytosolic lipases and autophagy factors to gain access to the lipids in the core of the lipid droplets.