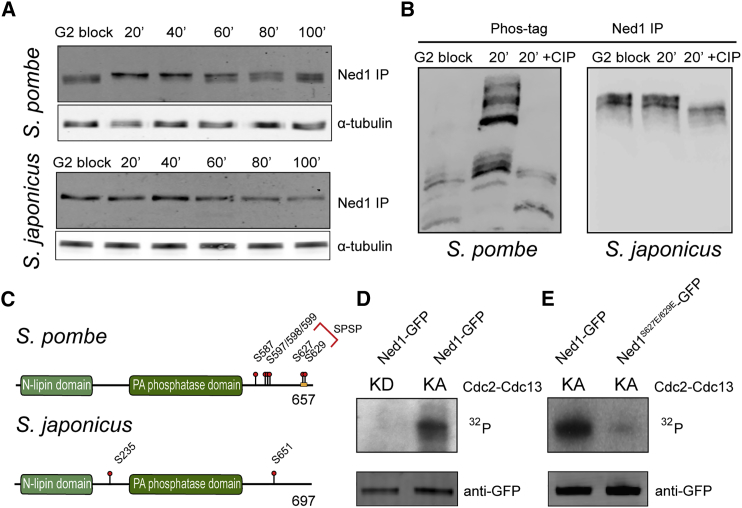
Figure 2.
The Lipin Ned1 Is Hyperphosphorylated During Mitosis in S. pombe, but Not in S. japonicus
(A) Cell-cycle synchronization experiments utilizing the temperature-sensitive mutant alleles of cdc25 (cdc25-22 for S. pombe and cdc25-D9 for S. japonicus). Time after release from the G2/M block is in minutes. Ned1-GFP from each time point was immunoprecipitated and subjected to WB analysis. WB for α-tubulin of input lysates was used as a loading control.
(B) Western blot analysis of immunoprecipitated Ned1-GFP from G2/M-blocked and mitotic cells (20 min after release) of S. pombe and S. japonicus. Samples were separated on 6% SDS-PAGE gel in the presence of Phos-tag. The mitotic samples were also subjected to CIP treatment.
(C) Schematic diagrams representing Ned1 S/T phosphorylation sites identified by LC-MS/MS analysis in S. pombe and S. japonicus. Positions of the evolutionarily conserved N-lipin and catalytic domains are also shown. The red bracket indicates a putative CDK1 phosphorylation site.
(D) CDK1 phosphorylates the S. pombe lipin Ned1 in vitro. Cdc2-Cdc13 kinase assays were performed using Ned1-GFP purified from G2-arrested cdc25-22 S. pombe cells. Ned1-GFP was incubated either with active (KA) or inactive (KD) Cdc2-Cdc13 kinase complexes. Half of the kinase reaction was used to detect phosphorylation by autoradiography (32P) and half was used in western blots with anti-GFP antibodies.
(E) Serine residues at positions 627 and 629 are essential for phosphorylation of Ned1 by Cdc2-Cdc13 in vitro. The kinase assays were performed using either wild-type or S627E/S629E mutant Ned1 proteins purified from cdc25-22 S. pombe cells arrested at G2/M transition.
See also Figure S2.