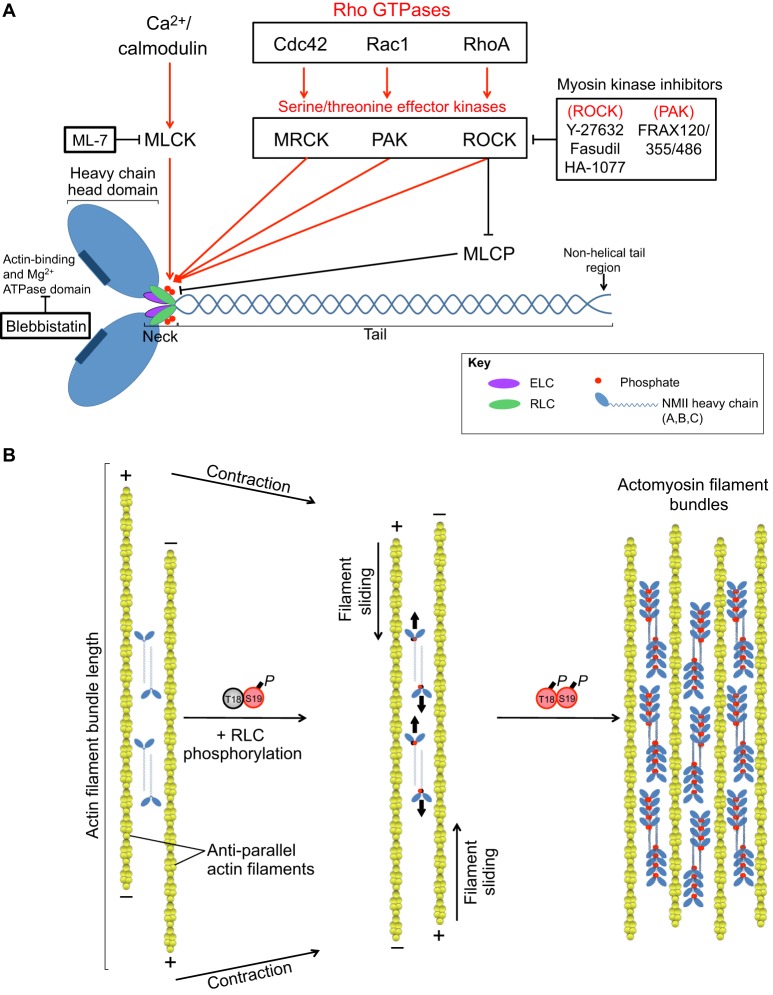
Fig. 1.
The structure of NMII and its regulation by serine/threonine kinases. (A) NMII consists of a heavy chain, which includes a globular head domain that binds both actin and ATP; a neck region, which binds both the essential and regulatory light chains (ELC and RLC, respectively); and a tail region, which homodimerizes in a helical fashion, as well as a non-helical tail region that directs the subcellular localization of the NMII isoform. Serine/threonine kinases regulate NMII activity by phosphorylation of the myosin RLC on residues Thr18 and Ser19. These kinases function downstream of small Rho GTPases, as well as downstream of Ca2+/calmodulin signaling pathways. This figure also indicates pharmacological inhibitors of myosin regulatory kinases that can be used to modulate NMII activity. (B) NMII filaments associate with each other in an anti-parallel fashion, allowing them to crosslink and slide actin filaments past each other. RLC Ser19 phosphorylation increases NMII ATPase activity, leading to contraction of actin filament bundles, and phosphorylation of both Ser19 and Thr18 increases NMII ATPase activity, driving the association of multiple actin filaments into actomyosin filament bundles, often referred to as stress fibers. MLCK, myosin light chain kinase; MRCK, myotonic dystrophy kinase-related Cdc42-binding kinase; PAK, p21-associated kinase; ROCK, RhoA-associated kinase; MLCP, myosin light chain phosphatase.