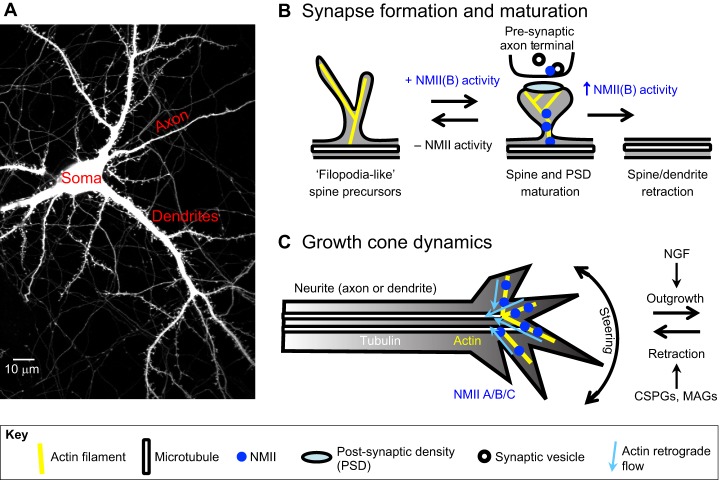
Fig. 2.
NMII regulates neuronal plasticity. (A) Confocal image of a GFP-expressing primary rat hippocampal neuron, highlighting the cell body, or soma, and processes, including post-synaptic dendrites, which form spines, and pre-synaptic axons, which form axon terminals containing synaptic vesicles. (B) NMII drives dynamic changes in neuronal morphology, including changes in dendritic spine formation and maturation, driven primarily by the isoform NMIIB. At the post-synaptic spine, NMII drives changes in actin organization that regulate spine and post-synaptic density (PSD) morphology and size, whereas, on the pre-synaptic side, NMII participates in synaptic vesicle recycling. The absence or inhibition of NMIIB activity results in dynamic ‘filopodia-like’ spine precursors and prevents spine maturation. In contrast, NMIIB activity drives spine and PSD maturation, although further NMIIB activity might result in spine and even dendrite retraction. (C) At the growth cone, all three NMII isoforms are present, and regulate substrate attachment and actin retrograde flow underlying neurite outgrowth. NGF, nerve growth factor; CSPGs, chondroitin sulfate proteoglycans; MAGs, myelin-associated glycoproteins.