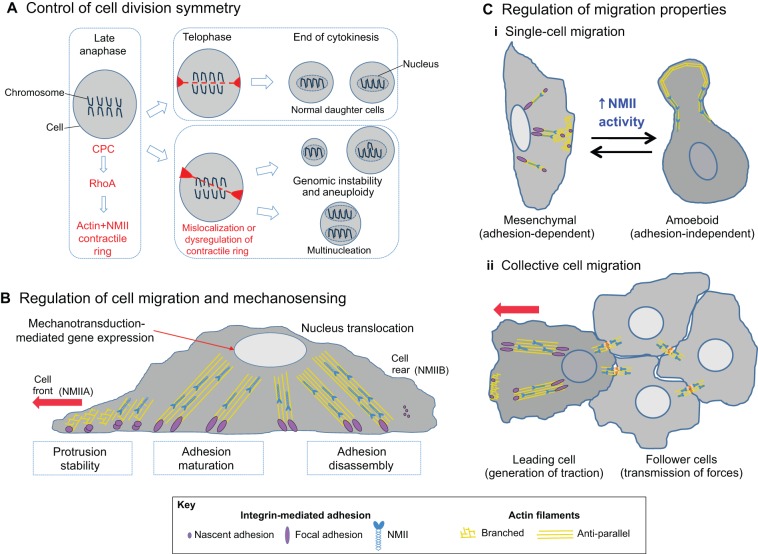
Fig. 3.
NMII drives cancer cell progression. (A) NMII in cell division. At the end of anaphase during mitosis, the chromosomal passenger complex (CPC) induces RhoA-mediated formation of an actin-NMII contractile ring (red), resulting in cytokinesis and division into two daughter cells (upper box; telophase). However, mislocalization of the contractile ring (lower box) can result in genetic abnormalities that are linked to cancer, such as aneuploidy or multinucleation. (B) NMII in cell migration and metastasis. During adhesion-dependent cell migration (see Box 1), cells polymerize actin at the cell front (unaligned yellow lines), while integrin-based adhesions (purple circles) mediate attachment to the extracellular matrix. NMIIA (blue) generates forces that alter actin organization at the cell front and initiate adhesion maturation (purple ellipses indicate adhesion elongation). NMIIB (blue) is involved in the formation of stress fibers (aligned yellow lines), nucleus translocation and in the detachment of adhesions at the cell rear. These mechanical forces induced by NMII can also influence other biochemical pathways (mechanotransduction) that are able to modify cell behavior. (C) NMII participates in various modes of cell migration. (i) Invasion of a single tumor cell is either mesenchymal (adhesion-dependent) or amoeboid (adhesion-independent). For mesenchymal-like, single-cell migration, myosin regulates the migration process as described in B; for amoeboid-like single-cell migration, increased NMII-mediated tension affects the cortical actin network (Ruprecht et al., 2015), allowing for adhesion-independent migration through porous matrices. (ii) During collective cell migration, the leading cell generates NMII-mediated traction forces that are propagated to the follower cells through cell–cell adhesions (see Fig. 4 for details).