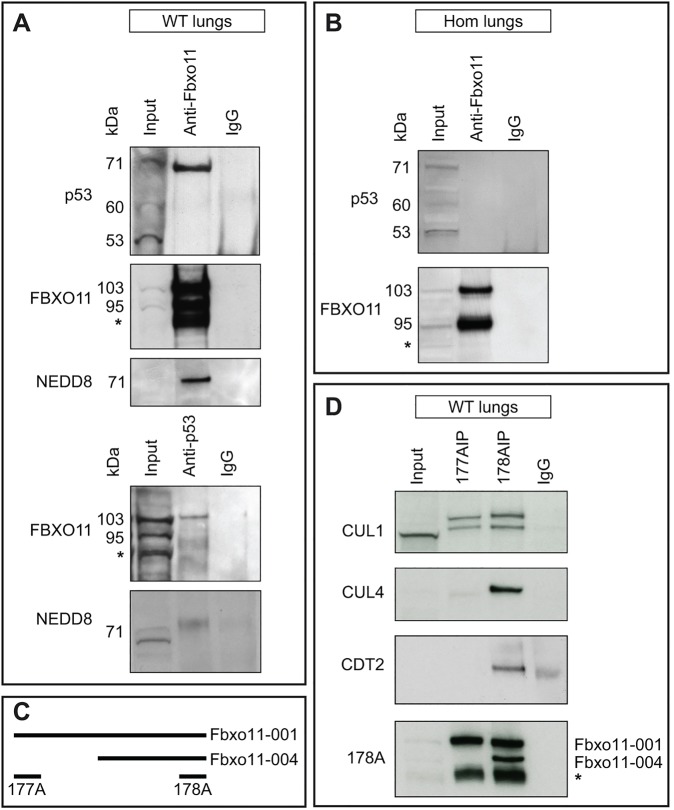
Fig. 3.
Interaction of FBXO11 with p53. (A) Protein lysate from E15.5 wild-type embryonic lungs was used for immunoprecipitation using an anti-FBXO11 (top, second and third panel) or an anti-p53 (fourth and fifth panel) antibody. Normal rabbit IgG was used as a control (IgG). Western blots were probed with antibodies as indicated: p53 (panel 1); FBXO11 (178A which detects both large and small isoforms of FBXO11, 103 and 95 kDa; panel 2 and 4); NEDD8 (panel 3 and 5). A degradation band observed with FBXO11 is indicated by an asterisk. (B) Protein lysate from E15.5 Jeff homozygote (Hom) lungs was used for immunoprecipitation using an anti-FBXO11 antibody (178A). Normal rabbit IgG was used as a control (IgG). The top panel was probed with an anti-p53 antibody, the bottom with an anti-FBXO11 antibody (178A). (C) Alignment of the fragments of FBXO11 protein used to produce the 177A and 178A antibodies for FBXO11. The epitope recognized by 177A maps to a region between residue 1 and 50 of human FBXO11, which is exclusive to the large isoform of mouse FBXO11 (Fbxo11-001). The epitope recognized by 178A maps to a region between residue 877 and 927 of human FBXO11 and is found in both mouse isoforms (Fbxo11-001 and 004). (D) Protein lysate from E15.5 wild-type (WT) embryonic lungs was used for immunoprecipitation using two anti-FBXO11 antibodies: 177A, which detects the large isoform of mouse FBXO11 (Fbxo11-001, 930 aa, 103 kDa), and 178A, which detects both the large and small isoforms (Fbxo11-001, 930 aa, 103 kDa and Fbxo11-004, 855 aa, 95 kDa). The blots were probed with anti-CUL1, -CUL4, -CDT2 and 178A antibodies. A degradation band of FBXO11 is indicated by an asterisk.