Fig. 4.
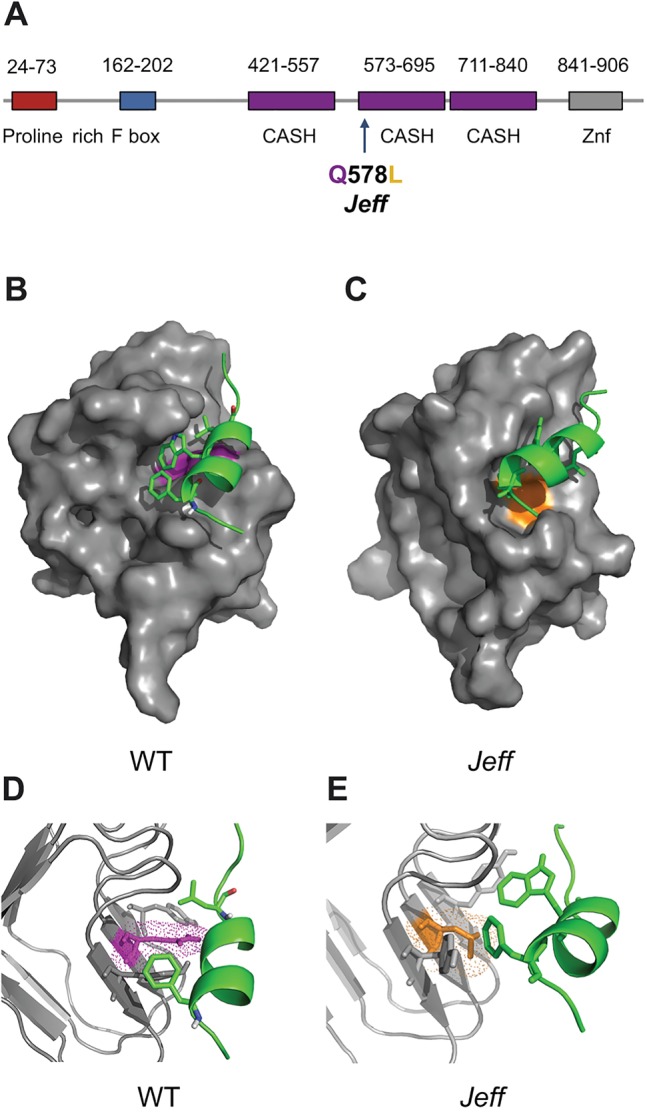
Predicted structure of the FBXO11-p53 complex. (A) Localization of the Jeff mutation (Q578L) in the predicted protein structure of FBXO11 on Ensembl. CASH, carbohydrate-binding proteins and sugar hydrolysis; Znf, zinc finger domain. (B-E) Cartoon representation of p53 (green) docked in the FBXO11 (grey) hydrophobic cleft to form the FBXO11-p53 complex. The wild-type (WT) complex is on the left with the wild-type residue (Q) coloured purple (B,D). The putative mutated complex found in the Jeff mutation is indicated on the right with the mutated amino acid (L) coloured orange (C,E). The mutated complex has an extended and distorted binding site, possibly contributing to the loss of binding to p53. Figure produced using The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC.
