Fig. 5.
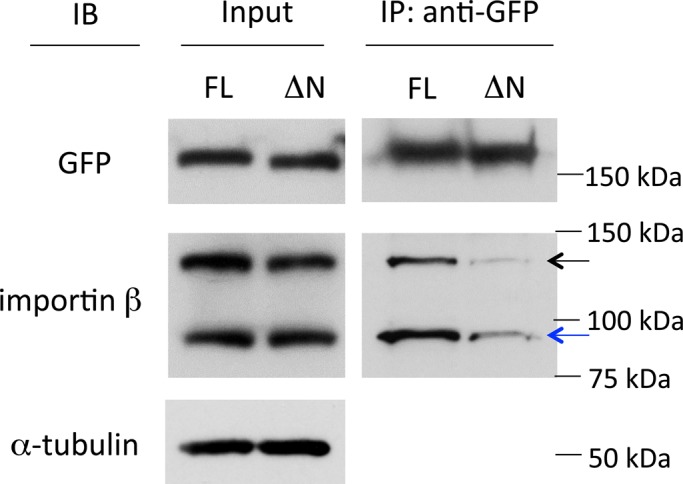
Interaction between mDia2 and importin β detected by immunoprecipitation (IP) assay. Cell lysate co-transfected with EGFP-mDia2 [full-length (FL) or 33-1171 aa (ΔN)] and mCherry-importin β were incubated with GFP antibody and protein G Sepharose beads. Portions of the input (whole cell lysate) and bound proteins from IP were separated by SDS-PAGE followed by immunoblotting (IB) using antibodies against GFP, importin β and α-tubulin. Black arrow indicates exogenous mCherry-importin β, and blue arrow indicates endogenous importin β. Full-length mDia2 pulled down both endogenous importin β and mCherry-importin β at a significantly higher level than mDia2 with an N-terminal truncation. α-tubulin was used as an internal control.
