Figure 2.
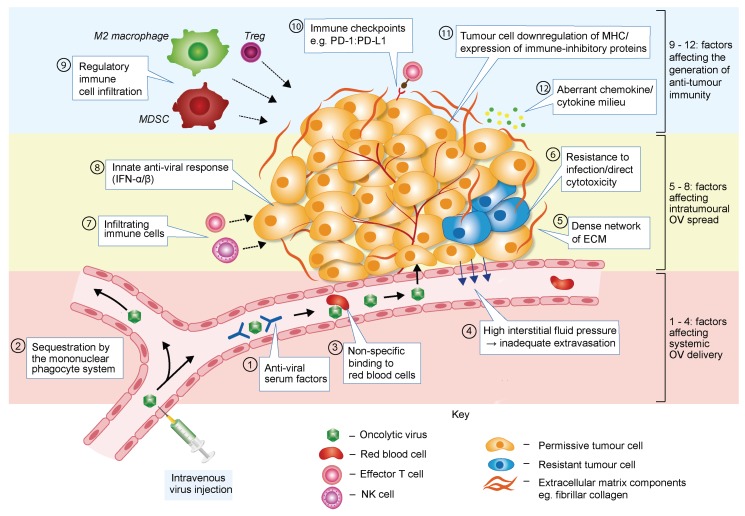
Barriers to effective OV therapy. When delivered to tumours via the bloodstream, oncolytic viruses (OVs) are vulnerable to anti-viral serum factors such as complement proteins and neutralising antibodies (box 1). Binding of these factors accelerates OV clearance from the circulation by macrophages in the liver, lungs and spleen (the mononuclear phagocyte system) (box 2). Systemic delivery of OVs may be further compromised by their non-specific binding to red blood cells (box 3). Once the target site is reached, an additional hurdle is posed by high interstitial fluid pressure within the tumour, which disfavours OV extravasation from the vasculature (box 4). Following extravasation, or after intratumoural OV injection, several factors may limit intratumoural viral spread and therapeutic effectiveness. Abundant extracellular matrix (ECM) and high interstitial fluid pressure represent physical barriers to OV spread by impeding virus diffusion between cells (box 5). Cancer cells within a tumour are likely to be heterogeneous in their susceptibility to virus infection and capacity to support the OV life cycle, with some cells displaying residual intracellular anti-viral activity and/or resistance to OV-mediated cell killing (box 6). Some viruses may also bind normal interstitial cells with a similar affinity as tumour cells, resulting in OV sequestration away from the target cancer cell (not illustrated in the figure). Infiltrating and resident innate immune cells (such as NK cells or macrophages) and anti-viral T cells will also contribute to limit the magnitude of viral production and spread (box 7). In some tumour cells (or normal stromal cells), virus infection may also trigger an anti-viral immune response, leading to release of type I IFN and impediment of virus multiplication (box 8). The immune-tolerant tumour microenvironment may hamper OV-induced anti-cancer immune responses in multiple ways (boxes 9–12), thereby limiting the efficacy of OV-based therapies.